Search Thermo Fisher Scientific
Thermo Scientific Chemicals
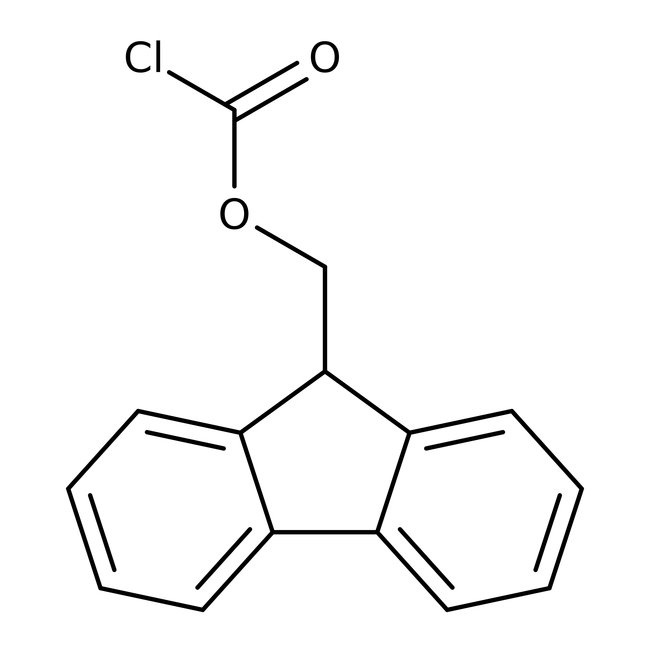
9-Fluorenylmethyl chloroformate, 98+%, Thermo Scientific Chemicals
Store cold
Catalog number ALFA11683.06
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
5 g
Specifications
Chemical Name or Material9-Fluorenylmethyl Chloroformate
CAS28920-43-6
Health Hazard 1H302+H312+H332-H314-H335
Health Hazard 2GHS H Statement
H314-H318-H302-H332
H314-H318-H302-H332
Health Hazard 3P260-P264b-P270-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P501c
View more
Base sensitive amino protecting group for solid-phase peptide synthesis9-Fluorenylmethyl chloroformate is used in capillary electrophoresis. It acts as a reagent in the precolumn derivatization of amines for HPLC and fluorescent detection. Further, it is used for derivatizing amino acids for HPLC analysis. In addition to this, it is used to prepare N-Fmoc amino acids for solid-phase peptide synthesis and oligonucleotide synthesis.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Base sensitive amino protecting group for solid-phase peptide synthesis9-Fluorenylmethyl chloroformate is used in capillary electrophoresis. It acts as a reagent in the precolumn derivatization of amines for HPLC and fluorescent detection. Further, it is used for derivatizing amino acids for HPLC analysis. In addition to this, it is used to prepare N-Fmoc amino acids for solid-phase peptide synthesis and oligonucleotide synthesis.
Solubility
Soluble in dioxane.
Notes
Moisture sensitive. Store in a cool place. Incompatible with alcohols and amines.
Base sensitive amino protecting group for solid-phase peptide synthesis9-Fluorenylmethyl chloroformate is used in capillary electrophoresis. It acts as a reagent in the precolumn derivatization of amines for HPLC and fluorescent detection. Further, it is used for derivatizing amino acids for HPLC analysis. In addition to this, it is used to prepare N-Fmoc amino acids for solid-phase peptide synthesis and oligonucleotide synthesis.
Solubility
Soluble in dioxane.
Notes
Moisture sensitive. Store in a cool place. Incompatible with alcohols and amines.
RUO – Research Use Only
General References:
- Reagent for the protection of amino groups in peptide synthesis (see Appendix 6) as their 9-fluorenylmethoxycarbonyl (Fmoc) derivatives: J. Org. Chem., 37, 3404 (1972); J. Am. Chem. Soc., 96, 4987 (1974); 99, 7363 (1977). Review: Acc. Chem. Res., 20, 401 (1987). They are particularly applicable in solid-phase peptide synthesis. The stability of Fmoc-protected amino acids to acidic conditions permits their conversion in many cases to the acid chlorides as active intermediates for peptide coupling, resistant to racemization, in contrast to other protected amino acid chlorides. For a review of peptide synthesis via amino acid halides, see: Acc. Chem. Res., 29, 268 (1996).
- In the presence of triethylamine, reacts with Pentafluorophenol, A15574 , to give the PFP carbonate, a useful active ester for the preparation of Fmoc-amino acids. Moreover, the active PFP ester of the protected amino acid can be obtained by in situ DCC coupling with the liberated PFP: Synthesis, 303 (1986).
- Cleavage of the Fmoc group occurs under mildly basic conditions:
- Ethanolamine: J. Am. Chem. Soc., 92, 5748 (1970); J. Org. Chem., 37, 3404 (1972). Piperidine: J. Org. Chem., 52, 1197 (1987); applicable to solid-phase peptide synthesis.
- TBAF in DMF; rapid reaction at room temperature: Tetrahedron Lett., 28, 6617 (1987). For both the deblocking of Fmoc-protected amino acids and for the removal of excess reagent during the protection step, 4-(Aminomethyl) piperidine, L11577 , has been recommended: J. Org. Chem., 51, 3732 (1986); 55, 721 (1990), particularly in conjunction with Fmoc-protected acid chlorides as the active species. Even better results have been obtained with Tris(2-aminoethyl) amine, B21789 , in this type of chemistry: J. Org. Chem., 55, 1673 (1990).