Search Thermo Fisher Scientific
Thermo Scientific Chemicals
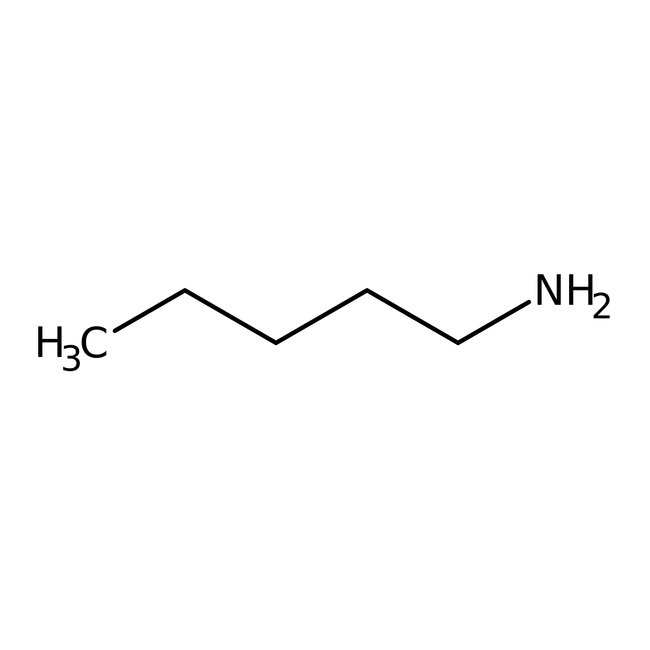
1-Pentylamine, 98%, Thermo Scientific Chemicals
CAS: 110-58-7 | C5H13N | 87.166 g/mol
Catalog number ALFA11674.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or Material1-Pentylamine
CAS110-58-7
Health Hazard 1H225-H302+H312-H314-H331-H335
Health Hazard 2GHS H Statement
H225-H331-H314-H318-H302-H312
Highly flammable liquid and vapor.
Toxic if inhaled.
Causes severe skin burns and eye damage.
Causes serious eye damage.
Harmful if swallowed.
Harmful in contact with skin.
H225-H331-H314-H318-H302-H312
Highly flammable liquid and vapor.
Toxic if inhaled.
Causes severe skin burns and eye damage.
Causes serious eye damage.
Harmful if swallowed.
Harmful in contact with skin.
Health Hazard 3P210-P233-P235-P240-P241-P242-P243-P260-P264b-P270-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P370+P378q-P501c
View more
1-Pentylamine is used as a solvent, as a raw material in the manufacture of a variety of other compounds, including dyes, emulsifiers, and pharmaceutical products and as a flavoring agent.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-Pentylamine is used as a solvent, as a raw material in the manufacture of a variety of other compounds, including dyes, emulsifiers, and pharmaceutical products and as a flavoring agent.
Solubility
Soluble in alcohol, ether.
Notes
Air Sensitive. Store away from strong oxidizing agents. keep container tightly closed. Store in cool, dry conditions in well sealed containers.
1-Pentylamine is used as a solvent, as a raw material in the manufacture of a variety of other compounds, including dyes, emulsifiers, and pharmaceutical products and as a flavoring agent.
Solubility
Soluble in alcohol, ether.
Notes
Air Sensitive. Store away from strong oxidizing agents. keep container tightly closed. Store in cool, dry conditions in well sealed containers.
RUO – Research Use Only
Patrik Spanel.; David Smith. Selected ion flow tube studies of the reactions of H3O+, NO+, and O2+ with eleven amine structural isomers of c5h13n1. International Journal of Mass Spectrometry. 1999, 185-187, 139-147.