Search Thermo Fisher Scientific
Thermo Scientific Chemicals
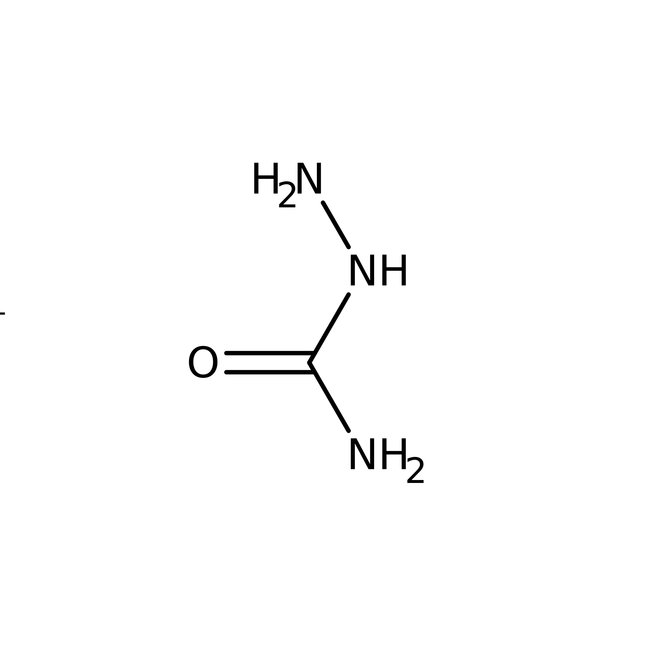
Semicarbazide hydrochloride, 99%, Thermo Scientific Chemicals
Urease substrate and MAO inhibitor
| Catalog Number | Quantity |
|---|---|
| ALFA11668.22 | 100 g |
Catalog number ALFA11668.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Chemical Name or MaterialSemicarbazide hydrochloride
CAS563-41-7
Health Hazard 1H301-H315-H319-H351
Health Hazard 2GHS H Statement
H301-H315-H319-H335
Toxic if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H301-H315-H319-H335
Toxic if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P201-P202-P264b-P270-P280-P280i-P301+P310-P302+P352-P305+P351+P338-P308+P313-P310-P330-P332+P313-P362-P501c
View more
Semicarbazide hydrochloride is used as urease substrate and MAO inhibitor. Derivatizing agent for carbonyl compounds as their semicarbazones which produces crystalline compounds with characteristic melting points. Also used in heterocyclic synthesis. Semicarbazone formation has been used to separate carbonyl compounds from mixtures by adsorption onto silica gel from a hydrocarbon solution. Regeneration is by hydrolysis with oxalic acid.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Semicarbazide hydrochloride is used as urease substrate and MAO inhibitor. Derivatizing agent for carbonyl compounds as their semicarbazones which produces crystalline compounds with characteristic melting points. Also used in heterocyclic synthesis. Semicarbazone formation has been used to separate carbonyl compounds from mixtures by adsorption onto silica gel from a hydrocarbon solution. Regeneration is by hydrolysis with oxalic acid.
Solubility
Soluble in water (>100g/L).
Notes
Store in cool, dry conditions in well sealed container. Store away from oxidizing agent.
Semicarbazide hydrochloride is used as urease substrate and MAO inhibitor. Derivatizing agent for carbonyl compounds as their semicarbazones which produces crystalline compounds with characteristic melting points. Also used in heterocyclic synthesis. Semicarbazone formation has been used to separate carbonyl compounds from mixtures by adsorption onto silica gel from a hydrocarbon solution. Regeneration is by hydrolysis with oxalic acid.
Solubility
Soluble in water (>100g/L).
Notes
Store in cool, dry conditions in well sealed container. Store away from oxidizing agent.
RUO – Research Use Only
General References:
- Phedias Diamandis et. al. Chemical genetics reveals a complex functional ground state of neural stem cells. Nature Chemical Biology. 2007, 3 (5), 268-273.
- Fumiko Marttila-Ichihara; Karolien Castermans; Kaisa Auvinen; Mirjam G A Oude Egbrink; Sirpa Jalkanen; Arjan W Griffioen; Marko Salmi. Small-molecule inhibitors of vascular adhesion protein-1 reduce the accumulation of myeloid cells into tumors and attenuate tumor growth in mice. Journal of Immunology. 2010, 184 (6), 3164-3173.
- Derivatizing agent for carbonyl compounds as their semicarbazones. Also used in heterocyclic synthesis. Semicarbazone formation has been used to separate carbonyl compounds from mixtures by adsorption onto silica gel from a hydrocarbon solution. Regeneration is by hydrolysis with oxalic acid: Tetrahedron, 37, 843 (1981).
- Other reagents for the cleavage of semicarbazones include: Amberlyst™ 15 in aqueous acetone: J .Chem. Soc., Perkin 1, 2563 (1988); Dowex™ 50 in aqueous suspension: J. Org. Chem., 53, 878 (1988); CuSO4 in aqueous THF: J. Prakt. Chem., 322 1063 (1980).