Search Thermo Fisher Scientific
Thermo Scientific Chemicals
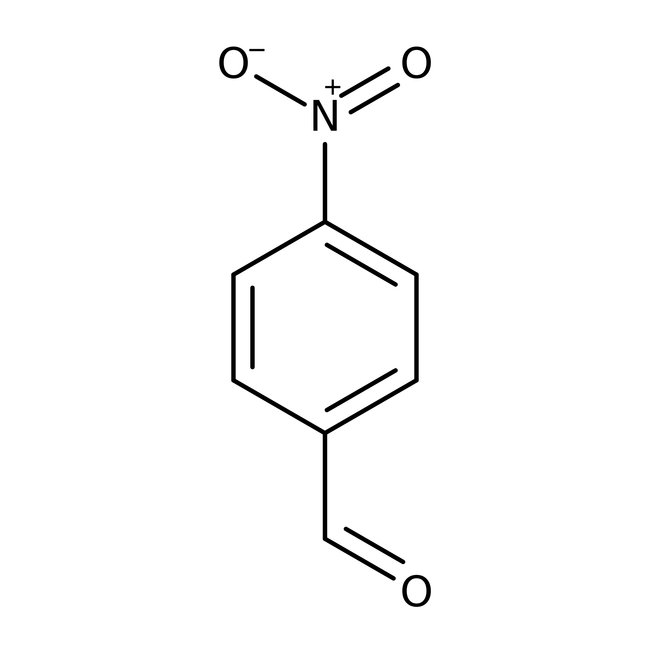
4-Nitrobenzaldehyde, 99%, Thermo Scientific Chemicals
CAS: 555-16-8 | C7H5NO3 | 151.12 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA11655.14 | 25 g |
Catalog number ALFA11655.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or Material4-Nitrobenzaldehyde
CAS555-16-8
Health Hazard 1H317-H319
Health Hazard 2GHS H Statement
H319-H317
Causes serious eye irritation.
May cause an allergic skin reaction.
H319-H317
Causes serious eye irritation.
May cause an allergic skin reaction.
Health Hazard 3P261-P264b-P272-P280-P302+P352-P305+P351+P338-P333+P313-P363-P501c
View more
4-Nitrobenzaldehyde is used in the preparation of homoallylic alcohols. It is also involved in the development and evaluation of a series of tripeptide organocatalysts.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Nitrobenzaldehyde is used in the preparation of homoallylic alcohols. It is also involved in the development and evaluation of a series of tripeptide organocatalysts.
Solubility
Soluble in water, ethanol, benzene, glacial acetic acid. Slightly soluble in ether.
Notes
Incompatible with strong oxidizing agents, strong bases and strong reducing agents.
4-Nitrobenzaldehyde is used in the preparation of homoallylic alcohols. It is also involved in the development and evaluation of a series of tripeptide organocatalysts.
Solubility
Soluble in water, ethanol, benzene, glacial acetic acid. Slightly soluble in ether.
Notes
Incompatible with strong oxidizing agents, strong bases and strong reducing agents.
RUO – Research Use Only
General References:
- 1,3-Oxathiolanes (see 2-Mercaptoethanol, A15890) can be selectively cleaved in the presence of dithioacetals using 4-nitrobenzaldehyde catalyzed by TMS-OTf: J. Chem. Soc., Chem. Commun., 1937 (1994). This combination has been used for conversion of thioketones to ketones: Tetrahedron Lett., 36, 2277 (1995).
- Kapoor, M.; Majumder, A. B.; Gupta, M. N. Promiscuous Lipase-Catalyzed C-C Bond Formation Reactions Between 4 Nitrobenzaldehyde and 2-Cyclohexen-1-one in Biphasic Medium: Aldol and Morita-Baylis-Hillman Adduct Formations. Catal. Lett. 2015, 145 (2), 527-532.
- Verma, N.; Kundi, V.; Ahmed, N. Piperidine-mediated annulation of 2-acylphenols with 4-nitrobenzaldehyde to 3-benzofuranones. Tetrahedron Lett. 2015, 56 (28), 4175-4179.