Search Thermo Fisher Scientific
Thermo Scientific Chemicals
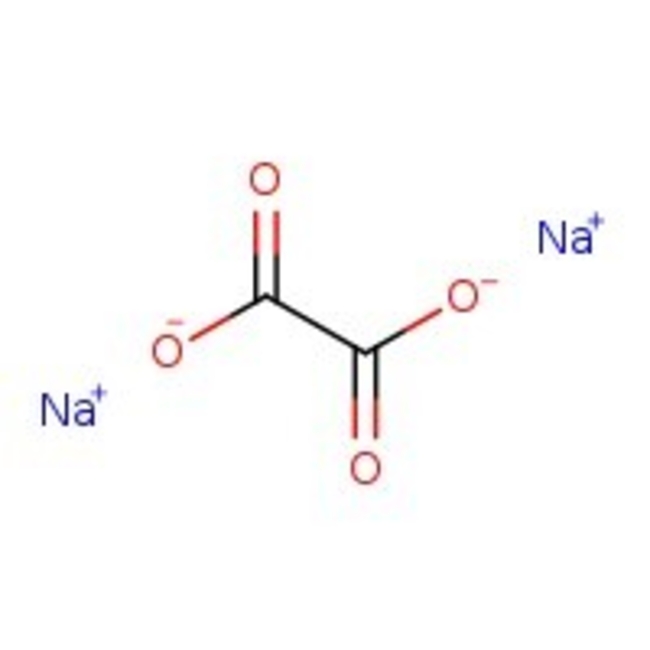
Sodium oxalate, 99%, Thermo Scientific Chemicals
CAS: 62-76-0 | C2Na2O4 | 134.00 g/mol
Catalog number ALFA11648.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS108-75-8
IUPAC Name2,4,6-trimethylpyridine
Molecular FormulaC8H11N
InChI KeyBWZVCCNYKMEVEX-UHFFFAOYSA-N
SMILESCC1=CC(C)=NC(C)=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear, colorless to yellow
Assay (GC)≥98.5%
Identification (FTIR)Conforms
CommentMay darken on storage
Refractive Index1.4965-1.5005 @ 20?C
View more
Sodium oxalate is used as reducing agent, as a primary standard for standardizing potassium permanganate solutions and as a neutralizer for exothermic acidic reactions. It finds application in metal cleaning preparations, metal extraction and separation, blueprint coatings, electroplating baths, special cement manufacturing, plant nutrition as well as in textile and pharmaceutical industries. It plays an important role to remove calcium ions from blood plasma and prevents blood clotting.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Sodium oxalate is used as reducing agent, as a primary standard for standardizing potassium permanganate solutions and as a neutralizer for exothermic acidic reactions. It finds application in metal cleaning preparations, metal extraction and separation, blueprint coatings, electroplating baths, special cement manufacturing, plant nutrition as well as in textile and pharmaceutical industries. It plays an important role to remove calcium ions from blood plasma and prevents blood clotting.
Solubility
Soluble in water and formic acid. Insoluble in ether and alcohol.
Notes
Hygroscopic. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents.
Sodium oxalate is used as reducing agent, as a primary standard for standardizing potassium permanganate solutions and as a neutralizer for exothermic acidic reactions. It finds application in metal cleaning preparations, metal extraction and separation, blueprint coatings, electroplating baths, special cement manufacturing, plant nutrition as well as in textile and pharmaceutical industries. It plays an important role to remove calcium ions from blood plasma and prevents blood clotting.
Solubility
Soluble in water and formic acid. Insoluble in ether and alcohol.
Notes
Hygroscopic. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Cao, S.; Zeng, W.; Li, T.; Gong, J.; Zhu, Z. Hydrothermal synthesis of NiO nanobelts and the effect of sodium oxalate. Mater. Lett. 2015, 156, 25-27.
- Fu, W.; Vaughan, J.; Gillespie, A. In situ AFM investigation of heterogeneous nucleation and growth of sodium oxalate on industrial gibbsite surfaces in concentrated alkaline solution. Chem. Eng. Sci. 2015, 126, 399-405.