Search Thermo Fisher Scientific
Thermo Scientific Chemicals
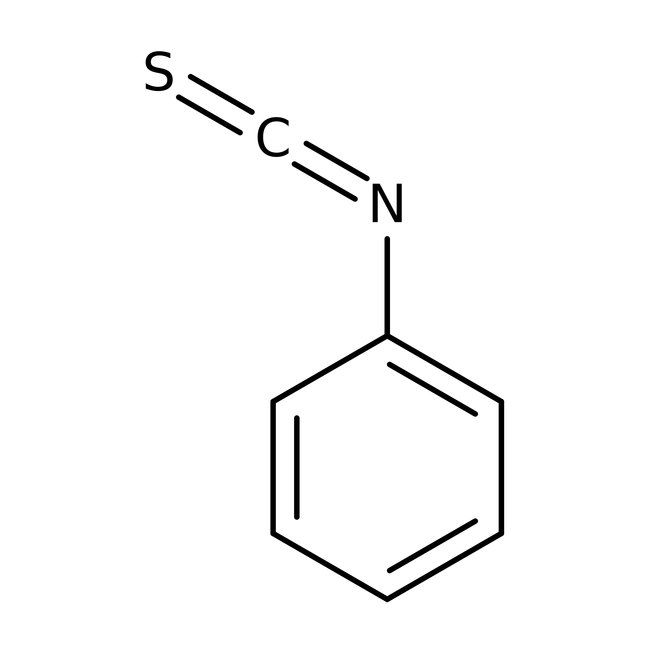
Phenyl isothiocyanate, 97%, Thermo Scientific Chemicals
CAS: 103-72-0 | C7H5NS | 135.18 g/mol
Catalog number ALFA11596.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Chemical Name or MaterialPhenyl isothiocyanate
CAS103-72-0
Health Hazard 1H227-H301+H311+H331-H314-H317-H334-H335-H361d
Health Hazard 2GHS H Statement
H301-H334-H314-H318-H227-H317
Toxic if swallowed.
May cause allergy or asthma symptoms or breathing difficulties if inhaled.
Causes severe skin burns and eye damage.
Causes serious eye damage.
Combustible liquid.
May cause an allergic skin reaction.
H301-H334-H314-H318-H227-H317
Toxic if swallowed.
May cause allergy or asthma symptoms or breathing difficulties if inhaled.
Causes severe skin burns and eye damage.
Causes serious eye damage.
Combustible liquid.
May cause an allergic skin reaction.
Health Hazard 3P201-P202-P210-P235-P260-P264b-P270-P271-P272-P280g-P281-P285-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P333+P313-P363-P370+P378q-P501c
View more
Phenyl isothiocyanate acts as a derivatizing reagent for primary and secondary amines. It is used in sequencing peptides by Edman degradation and in amino acid analyses by HPLC. It is used for derivatizing N-terminal amino acids of proteins for automated sequential analysis. It is a synthon for dithiadiazafulvalenes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Phenyl isothiocyanate acts as a derivatizing reagent for primary and secondary amines. It is used in sequencing peptides by Edman degradation and in amino acid analyses by HPLC. It is used for derivatizing N-terminal amino acids of proteins for automated sequential analysis. It is a synthon for dithiadiazafulvalenes.
Solubility
Soluble in alcohol, and ether. Insoluble in water.
Notes
Moisture Sensitive. Protect from humidity and water. Store under dry inert gas away from oxidizing agents and moisture. Store at 4°C.
Phenyl isothiocyanate acts as a derivatizing reagent for primary and secondary amines. It is used in sequencing peptides by Edman degradation and in amino acid analyses by HPLC. It is used for derivatizing N-terminal amino acids of proteins for automated sequential analysis. It is a synthon for dithiadiazafulvalenes.
Solubility
Soluble in alcohol, and ether. Insoluble in water.
Notes
Moisture Sensitive. Protect from humidity and water. Store under dry inert gas away from oxidizing agents and moisture. Store at 4°C.
RUO – Research Use Only
General References:
- F Lai; T Sheehan. Matrix effects in the derivatization of amino acids with naphthalene dicarboxaldehyde, 9-fluorenylmethyl chloroformate and phenylisothiocyanate. BioTechniques.1993, 14 (4), 642-649.
- Mark C Wesley; Luis M Pereira; Laurie A Scharp; Sitaram M Emani; Francis X McGowan; James A DiNardo. Pharmacokinetics of tranexamic acid in neonates, infants, and children undergoing cardiac surgery with cardiopulmonary bypass. Anesthesiology.2015, 122 (4), 746-758.
- Derivatizing agent for amino groups. Introduced by Edman for the determination of amino acid sequence by conversion to the thiourea, acid cleavage of the peptide bond and alkaline hydrolysis of the derived thiohydantoin: Acta Chem. Scand., 4, 277 (1950); Arkiv. Kemi., 14, 291 (1959). The thiourea can also be cleaved with TFA: Nature, 227, 716 (1970), or by peracid oxidation: Chem. Ber., 89, 2288 (1956).
- For general reactions of isothiocyanates, see Appendix 3.