Search Thermo Fisher Scientific
Thermo Scientific Chemicals
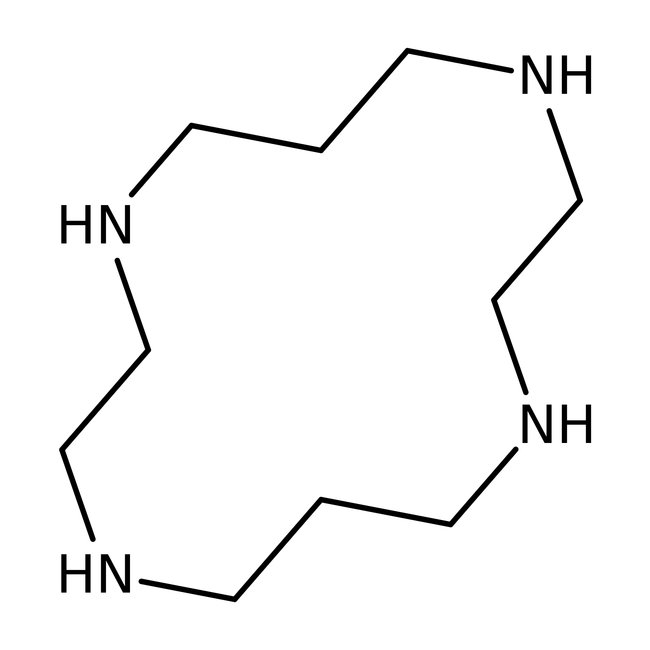
1,4,8,11-Tetraazacyclotetradecane, 98+%, Thermo Scientific Chemicals
CAS: 295-37-4 | C10H24N4 | 200.33 g/mol
Catalog number ALFA11516.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or Material1,4,8,11-Tetraazacyclotetradecane
CAS295-37-4
Health Hazard 1H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P501c
View more
1,4,8,11-Tetraazacyclotetradecane is used in the synthesis of molecules with electroactive cavities. It acts as a nitrogen crown ether analogue and as an antioxidant in rubber. It is also used in the preparation of plerixafor derivatives.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,4,8,11-Tetraazacyclotetradecane is used in the synthesis of molecules with electroactive cavities. It acts as a nitrogen crown ether analogue and as an antioxidant in rubber. It is also used in the preparation of plerixafor derivatives.
Solubility
Soluble in water.
Notes
Hygroscopic. Incompatible with oxidizing agents.
1,4,8,11-Tetraazacyclotetradecane is used in the synthesis of molecules with electroactive cavities. It acts as a nitrogen crown ether analogue and as an antioxidant in rubber. It is also used in the preparation of plerixafor derivatives.
Solubility
Soluble in water.
Notes
Hygroscopic. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Nitrogen analogue of the crown ethers (see Appendix 2), forming stable complexes with metal ions: Inorg. Chem., 4,1102, 1109 (1965); Can. J. Chem., 48, 1481 (1970). For examples of use as a versatile ligand in coordination chemistry, see: Acc. Chem. Res., 11, 392 (1978); J. Chem. Soc., Chem. Commun., 1322 (1986); 1075 (1987); 156 (1988); Inorg. Chem., 26, 908 (1987); J. Am. Chem. Soc., 110, 3679 (1988).
- Complex with iron(II) triflate is an effective epoxidation catalyst: J. Am. Chem. Soc., 113, 7052 (1991).
- The Ni(II) complex catalyzes the novel electrochemical reaction of epoxides with carbon dioxide to give good yields of cyclic carbonates: J. Chem. Soc., Chem. Commun., 43 (1995). A similar system catalyzes the electrochemical intramolecular reductive cyclization of a series of o-halogenated aryl alkenes: Tetrahedron Lett., 36, 4429 (1995):
- For use in synthesis of molecules with electroactive cavities, see (Ferrocenyl methyl) trimethyl ammonium iodide, 39399.
- Misra, A. C.; Luker, K. E.; Durmaz, H.; Luker, G. D.; Lahann, J. CXCR4-Targeted Nanocarriers for Triple Negative Breast Cancers. Biomacromolecules 2015, 16 (8), 2412-2417.