Search Thermo Fisher Scientific
Thermo Scientific Chemicals
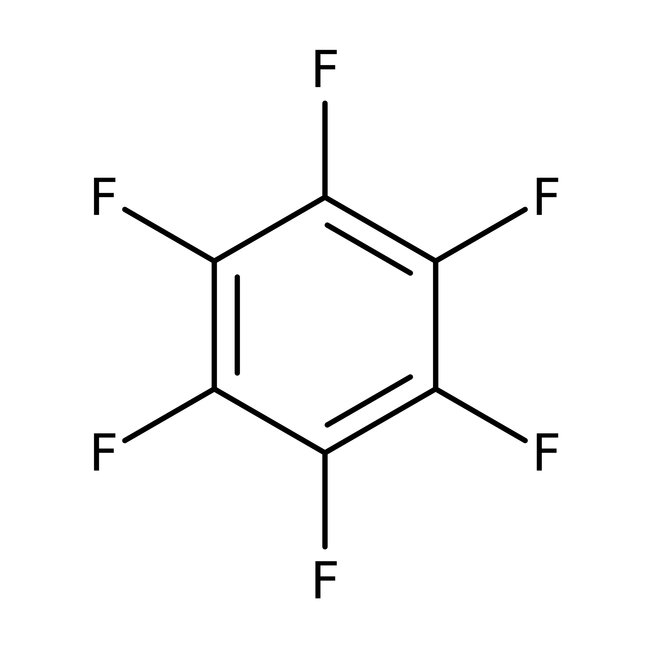
Hexafluorobenzene, 99%, Thermo Scientific Chemicals
CAS: 392-56-3 | C6F6 | 186.056 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA11500.09 | 10 g |
Catalog number ALFA11500.09
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
10 g
Chemical Identifiers
CAS7789-23-3
IUPAC Namepotassium fluoride
Molecular FormulaFK
InChI KeyNROKBHXJSPEDAR-UHFFFAOYSA-M
SMILES[F-].[K+]
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White
Appearance (Form)Powder
Assay35 to 45 wt % (Mass balance)
Water3 to 10 % (K.F.)
Hexafluorobenzene is used as a solvent in photochemical reactions. It is also used as a reference compound in fluorine-19 NMR, carbon-13 NMR. It is used as a solvent in proton NMR, IR spectrum and UV-spectra. It is used as anticorrosive, antifriction and anti-tumor agents. Further, it is used as a reference molecule to investigate tissue oxygenation studies. It forms series of 1:1 complexes with naphthalene, anthracene, phenanthrene, pyrene and triphenylene.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Hexafluorobenzene is used as a solvent in photochemical reactions. It is also used as a reference compound in fluorine-19 NMR, carbon-13 NMR. It is used as a solvent in proton NMR, IR spectrum and UV-spectra. It is used as anticorrosive, antifriction and anti-tumor agents. It forms series of 1:1 complexes with naphthalene, anthracene, phenanthrene, pyrene and triphenylene.
Solubility
Immiscible with water.
Notes
Incompatible with strong oxidizing agents.
Hexafluorobenzene is used as a solvent in photochemical reactions. It is also used as a reference compound in fluorine-19 NMR, carbon-13 NMR. It is used as a solvent in proton NMR, IR spectrum and UV-spectra. It is used as anticorrosive, antifriction and anti-tumor agents. It forms series of 1:1 complexes with naphthalene, anthracene, phenanthrene, pyrene and triphenylene.
Solubility
Immiscible with water.
Notes
Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Exchanges with Grignard reagents, under catalysis by CoCl2, to give the pentafluorophenyl Grignard reagent: J. Organomet. Chem., 19, 191 (1969).
- Reacts with ethyl cyanoacetate, in the presence of K2CO3 in DMF, to displace one F atom, as the first step of a synthesis of pentafluorophenylacetonitrile: Org. Synth. Coll., 6, 873 (1988).
- Gong, S.; Luo, Q.; Feng, X.; Li, Q.-s.; Xie, Y.; King, R. B.; Schaefer Iii, H. F. Triple decker sandwiches and related compounds of the first row transition metals with cyclopentadienyl and hexafluorobenzene rings: remarkable effects of fluorine substitution. Phys. Chem. Chem. Phys. 2015, 17 (31), 20100-20113.
- Ma, W. C.; Tsai, C. Y.; Huang, C. Investigation of atmospheric-pressure plasma deposited hexafluorobenzene fluorocarbon film. Surf. Coat. Technol. 2014, 259, 290-296.