Search Thermo Fisher Scientific
Thermo Scientific Chemicals
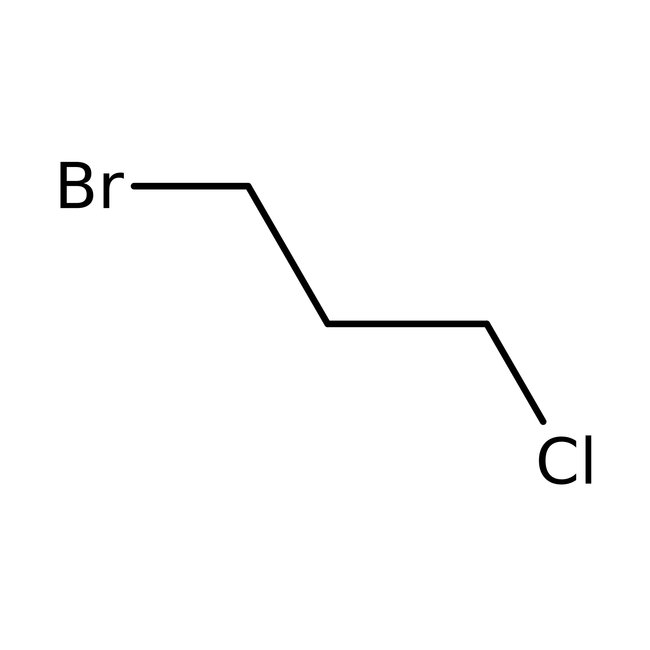
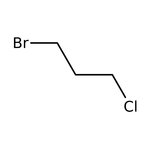
1-Bromo-3-chloropropane, 99%, Thermo Scientific Chemicals
CAS: 109-70-6 | C3H6BrCl | 157.435 g/mol
Catalog number ALFA11395.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Chemical Identifiers
CAS15115-60-3
IUPAC Name4-bromo-2,3-dihydro-1H-inden-1-one
Molecular FormulaC9H7BrO
InChI KeyUVVYFYLSZIMKMC-UHFFFAOYSA-N
SMILESBrC1=CC=CC2=C1CCC2=O
View more
Specifications Specification Sheet
Specification Sheet
Assay (HPLC)>96.0%
1-Bromo-3-chloropropane is used in the preparation active pharmaceutical ingredient intermediate such as gemfibrozil and reproterol. It is also involved in the preparation of cardiovascular diseases and analgesic drugs materials. It is utilized as a phase separation reagent for the isolation of ribonucleic acid (RNA) in high quality. It is considered as a replacement of chloroform in nucleic acid separations.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-Bromo-3-chloropropane is used in the preparation active pharmaceutical ingredient intermediate such as gemfibrozil and reproterol. It is also involved in the preparation of cardiovascular diseases and analgesic drugs materials. It is utilized as a phase separation reagent for the isolation of ribonucleic acid (RNA) in high quality. It is considered as a replacement of chloroform in nucleic acid separations.
Solubility
Miscible with alcohols, chlorofom and ether. Immiscible with water.
Notes
Incompatible with strong oxidizing, reducing agents, amines, nitrides, azo/diazo compounds, alkali metals and epoxides.
1-Bromo-3-chloropropane is used in the preparation active pharmaceutical ingredient intermediate such as gemfibrozil and reproterol. It is also involved in the preparation of cardiovascular diseases and analgesic drugs materials. It is utilized as a phase separation reagent for the isolation of ribonucleic acid (RNA) in high quality. It is considered as a replacement of chloroform in nucleic acid separations.
Solubility
Miscible with alcohols, chlorofom and ether. Immiscible with water.
Notes
Incompatible with strong oxidizing, reducing agents, amines, nitrides, azo/diazo compounds, alkali metals and epoxides.
RUO – Research Use Only
General References:
- Fan, X.; Sokorai, K. J. Formation of trichloromethane in chlorinated water and fresh-cut produce and as a result of reaction with citric acid. Postharvest Biol. Technol. 2015, 109, 65-72.
- Donnier-Maréchal, M.; Carato, P.; Le Broc, D.; Furman, C.; Melnyk, P. Synthesis and pharmacological evaluation of benzannulated derivatives as potent and selective sigma-1 protein ligands. Eur. J. Med. Chem. 2015, 92, 575-582.