Search Thermo Fisher Scientific
Thermo Scientific Chemicals
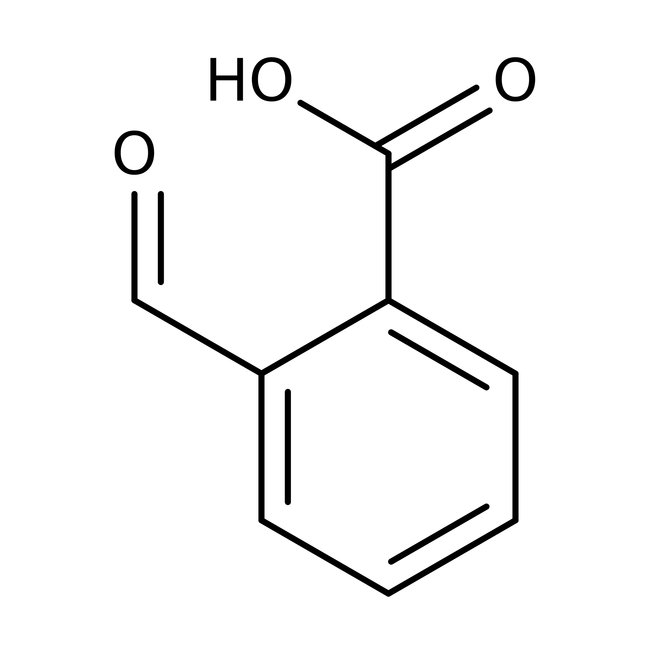
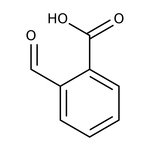
2-Carboxybenzaldehyde, 98+%, Thermo Scientific Chemicals
CAS: 119-67-5 | C8H6O3 | 150.133 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA11201.18 | 50 g |
Catalog number ALFA11201.18
Price (MYR)
386.00
Quantity:
50 g
Price (MYR)
386.00
Specifications
Chemical Name or Material2-Carboxybenzaldehyde
CAS119-67-5
Health Hazard 1H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P501c
View more
2-Carboxybenzaldehyde is a carboxylated derivative of benzaldehyde which is readily converted to 2-hydroxymethyl benzoic acid by CBA dehydrogenase. 2-Carboxybenzaldehyde is a metabolite of ampicillin phthalidyl ester. It has been used to synthesize a series of N-substituted isoindolinones by reductive C-N coupling and intramolecular amidation with amines.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Carboxybenzaldehyde is a carboxylated derivative of benzaldehyde which is readily converted to 2-hydroxymethyl benzoic acid by CBA dehydrogenase. 2-Carboxybenzaldehyde is a metabolite of ampicillin phthalidyl ester. It has been used to synthesize a series of N-substituted isoindolinones by reductive C-N coupling and intramolecular amidation with amines.
Solubility
Soluble in water.
Notes
Air Sensitive. Store away from air and oxidizing agents. Incompatible with strong bases.
2-Carboxybenzaldehyde is a carboxylated derivative of benzaldehyde which is readily converted to 2-hydroxymethyl benzoic acid by CBA dehydrogenase. 2-Carboxybenzaldehyde is a metabolite of ampicillin phthalidyl ester. It has been used to synthesize a series of N-substituted isoindolinones by reductive C-N coupling and intramolecular amidation with amines.
Solubility
Soluble in water.
Notes
Air Sensitive. Store away from air and oxidizing agents. Incompatible with strong bases.
RUO – Research Use Only
General References:
- Ka young lee; Jeong mi kim; Jae nyoung kim. Facile synthesis of 3-alkylidene-3H-isobenzofuranones from the Baylis-Hillman reaction of 2-carboxybenzaldehyde. Synlett. 2003, 357-360.
- KA Krasnov; VG Kartsev; EE Santarovich. Reaction of Barbituric, 2-Thiobarbituric Acids and Their Derivatives with 2-Carboxybenzaldehyde and Opianic Acid: Synthesis and Tautomerism of 5-(3'-Oxo-1',3'-dihydroisobenzofuran-1'-yl)barbituric Acids and Their 2-Thio Analogs. Chemistry of Heterocyclic Compounds. 2002, 38, 702-709.
- The aldehyde is in equilibrium with the cyclic lactol, which reacts with both alkyl and aryl Grignard reagents to give substituted phthalides: Tetrahedron, 44, 2903 (1988):