Search Thermo Fisher Scientific
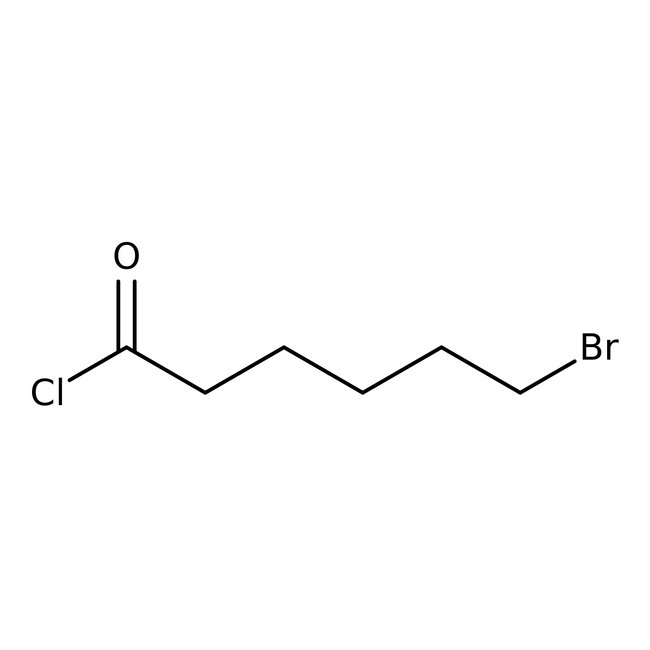
6-Bromohexanoyl chloride, 97%, Thermo Scientific Chemicals
Catalog number ALFA11063.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or Material6-Bromohexanoyl chloride
CAS22809-37-6
Health Hazard 1H314
Health Hazard 2GHS H Statement
H314-H318
Causes severe skin burns and eye damage.
Causes serious eye damage.
H314-H318
Causes severe skin burns and eye damage.
Causes serious eye damage.
Health Hazard 3P260-P264b-P280-P301+P330+P331-P303+P361+P353-P304+P340-P305+P351+P338-P310-P363-P501c
View more
6-Bromohexanoyl chloride is used as intermediates, organic synthesis, chemical research, agrochemicals and dyestuff.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
6-Bromohexanoyl chloride is used as intermediates, organic synthesis, chemical research, agrochemicals and dyestuff.
Solubility
Reacts with water.
Notes
Store in cool, dry conditions in well sealed containers. Keep container tightly closed. Moisture Sensitive
6-Bromohexanoyl chloride is used as intermediates, organic synthesis, chemical research, agrochemicals and dyestuff.
Solubility
Reacts with water.
Notes
Store in cool, dry conditions in well sealed containers. Keep container tightly closed. Moisture Sensitive
RUO – Research Use Only
General References:
- Koji Matsuoka,; Mikiko Terabatake,; Yosuke Saito,; Chiharu Hagihara,; Yasuaki Esumi,; Daiyo Terunuma. Hiroyoshi Kuzuhara. Synthesis of Carbosilane Compounds Functionalized with Three or Four β-Cyclodextrin Moieties. Use of a One-Pot Reaction in Liquid Ammonia for Birch Reduction and the Subsequent SN2 Replacement. Bulletin of the Chemical Society of Japan. 1998, 71(11),2709-2713.
- Yukito Murakami,; Akio Nakano,; Hidetsugu Ikeda.;Preparation of stable single-compartment vesicles with cationic and zwitterionic amphiphiles involving amino acid residues. J. Org. Chem. 1982, 47(11),2137-2144.