Search Thermo Fisher Scientific
Thermo Scientific Chemicals
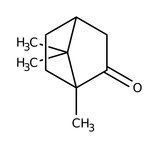
(±)-Camphor, 96%, Thermo Scientific Chemicals
CAS: 76-22-2 | C10H16O | 152.24 g/mol
Catalog number ALFA10936.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Chemical Name or Material(±)-Camphor
CAS76-22-2
Health Hazard 1H228-H302-H315-H319-H335
Health Hazard 2GHS H Statement
H228-H315-H319
Flammable solid.
Causes skin irritation.
Causes serious eye irritation.
H228-H315-H319
Flammable solid.
Causes skin irritation.
Causes serious eye irritation.
Health Hazard 3P210-P240-P241-P261-P264b-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P312-P330-P332+P313-P362-P370+P378q-P501c
View more
Camphor is used in the preparation of mothballs. It acts as a plasticizer for nitrocellulose, and an ingredient for fireworks and explosive munitions. It is useful in the treatment of sprains, swellings and inflammation. It is also used to synthesize carbon nanotubes by chemical vapor deposition process of camphor.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Camphor is used in the preparation of mothballs. It acts as a plasticizer for nitrocellulose, and an ingredient for fireworks and explosive munitions. It is useful in the treatment of sprains, swellings and inflammation. It is also used to synthesize carbon nanotubes by chemical vapor deposition process of camphor.
Solubility
Soluble in acetone, ethanol, diethylether, chloroform and acetic acid.
Notes
Incompatible with strong oxidizing agents, strong reducing agents and chlorinated solvents.
Camphor is used in the preparation of mothballs. It acts as a plasticizer for nitrocellulose, and an ingredient for fireworks and explosive munitions. It is useful in the treatment of sprains, swellings and inflammation. It is also used to synthesize carbon nanotubes by chemical vapor deposition process of camphor.
Solubility
Soluble in acetone, ethanol, diethylether, chloroform and acetic acid.
Notes
Incompatible with strong oxidizing agents, strong reducing agents and chlorinated solvents.
RUO – Research Use Only
General References:
- Ceacero-Vega, A. A.; Ballesteros, B.; Bejan, I.; Barnes, I.; Jiménez, E.; Albaladejo, J. Kinetics and Mechanisms of the Tropospheric Reactions of Menthol, Borneol, Fenchol, Camphor, and Fenchone with Hydroxyl Radicals (OH) and Chlorine Atoms (Cl). J. Phys. Chem. A 2012, 116 (16), 4097-4107.
- Rafiński, Z.; Kozakiewicz, A. Enantioselective Synthesis of Chromanones Bearing Quaternary Substituted Stereocenters Catalyzed by (1R)-Camphor-Derived N-Heterocyclic Carbenes. J. Org. Chem. 2015, 80 (15), 7468-7476.
- McLain, K. A.; Miller, K. A.; Collins, W. R. Introducing Organic Chemistry Students to Natural Product Isolation Using Steam Distillation and Liquid Phase Extraction of Thymol, Camphor, and Citral, Monoterpenes Sharing a Unified Biosynthetic Precursor. J. Chem. Educ. 2015, 92 (7), 1226-1228.