Search Thermo Fisher Scientific
Thermo Scientific Chemicals
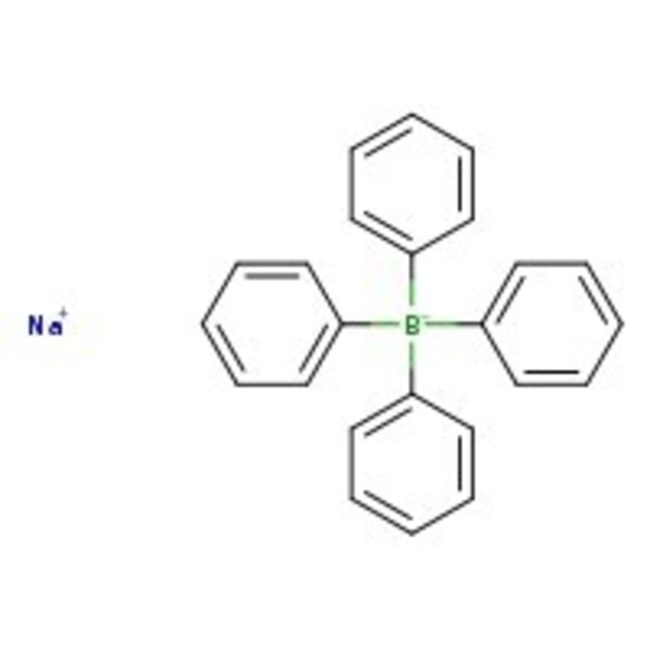
Sodium tetraphenylborate, 99%, Thermo Scientific Chemicals
CAS: 143-66-8 | C24H20BNa | 342.22 g/mol
Catalog number ALFA10909.06
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
5 g
Specifications
Chemical Name or MaterialSodium tetraphenylborate
CAS143-66-8
Health Hazard 1H301-H315-H319-H335
Health Hazard 2GHS H Statement
H301-H315-H319-H335
Toxic if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H301-H315-H319-H335
Toxic if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P270-P271-P280-P301+P310-P302+P352-P304+P340-P305+P351+P338-P312-P330-P332+P313-P362-P501c
View more
Sodium tetraphenylborate is used as a precipitating reagent for potassium and as a protecting agent to protect amino acids. It is used in the preparation of N-acylammonium salts. It finds application as a phenyl donor in palladium-catalyzed cross-coupling reactions to prepare arylalkenes and biaryl compounds. It is used in inorganic and organometallic chemistry due to its solubility in nonpolar solvents. Furthermore, it is involved in the isolation of complexes having dinitrogen ligands.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Sodium tetraphenylborate is used as a precipitating reagent for potassium and as a protecting agent to protect amino acids. It is used in the preparation of N-acylammonium salts. It finds application as a phenyl donor in palladium-catalyzed cross-coupling reactions to prepare arylalkenes and biaryl compounds. It is used in inorganic and organometallic chemistry due to its solubility in nonpolar solvents. Furthermore, it is involved in the isolation of complexes having dinitrogen ligands.
Solubility
Soluble in water and ethanol.
Notes
Light sensitive. Incompatible with strong acids and strong oxidizing agents.
Sodium tetraphenylborate is used as a precipitating reagent for potassium and as a protecting agent to protect amino acids. It is used in the preparation of N-acylammonium salts. It finds application as a phenyl donor in palladium-catalyzed cross-coupling reactions to prepare arylalkenes and biaryl compounds. It is used in inorganic and organometallic chemistry due to its solubility in nonpolar solvents. Furthermore, it is involved in the isolation of complexes having dinitrogen ligands.
Solubility
Soluble in water and ethanol.
Notes
Light sensitive. Incompatible with strong acids and strong oxidizing agents.
RUO – Research Use Only
General References:
- Reagent for potassium by precipitation: Anal. Chem., 29, 1044 (1957).
- Has been used to protect amino acids by formation of a diphenylborinate chelate, stable to AcOH or TFA, but cleaved by base: Tetrahedron, 39, 2995 (1983); Liebigs Ann. Chem., 127 (1989):
- The cross coupling of tetraphenylborates or aryl boronic acids with aryl halides is catalyzed by Pd(OAc)2 in aqueous solutions: Dokl. Chem. (Engl. Transl.), 315, 354 (1990); see also: Gazz. Chem. Ital., 120, 779 (1990). Cross couples with vinyl and aryl triflates under Pd catalysis: Tetrahedron Lett., 35, 4835 (1992); 33, 4815 (1992). Similarly, allyl acetates are phenylated: Tetrahedron Lett., 31, 7453 (1990).
- Howa, J. D.; Lott, M. J.; Ehleringer, J. R. Isolation and stable nitrogen isotope analysis of ammonium ions in ammonium nitrate prills using sodium tetraphenylborate. Rapid Commun. Mass Spectrom. 2014, 28 (13), 1530-1534.
- Boruń, A.; Trzcińska, I.; Bald, A. Conductometric Studies of Sodium Iodide, Sodium Tetraphenylborate, Tetrabutylammonium Iodide, and Sodium Tetrafluoroborate in 1-Propanol at Temperatures from (283.15 to 318.15) K. Int. J. Electrochem. Sci. 2014, 9, 7805-7818.