Search Thermo Fisher Scientific
Thermo Scientific Chemicals
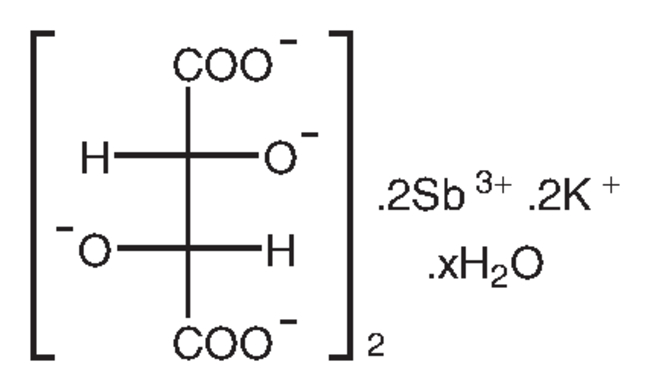
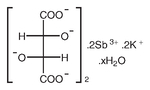
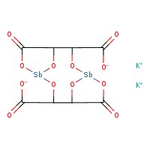
Antimony potassium tartrate hydrate, 98%, Thermo Scientific Chemicals
CAS: 331753-56-1 | C8H4K2O12Sb2 | 613.83 g/mol
Catalog number ALFA10889.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or MaterialAntimony potassium tartrate hydrate
CAS331753-56-1
Health Hazard 1H302+H332
Health Hazard 2GHS H Statement
H302-H332
Harmful if swallowed.
Harmful if inhaled.
H302-H332
Harmful if swallowed.
Harmful if inhaled.
Health Hazard 3P261-P264b-P270-P271-P301+P312-P304+P340-P312-P330-P501c
View more
Antimony potassium tartrate hydrate is a compound used in the treatment of schistosomiasis. It is also used in the textile industry to aid in binding certain dyes to fabrics. It finds application in medicine as an expectorant and a nauseant.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Antimony potassium tartrate hydrate is a compound used in the treatment of schistosomiasis. It is also used in the textile industry to aid in binding certain dyes to fabrics. It finds application in medicine as an expectorant and a nauseant.
Solubility
Soluble in water. Insoluble in ethanol.
Notes
Incompatible with mineral acids, strong bases, carbonates, lead, silver salts and strong oxidizing agents.
Antimony potassium tartrate hydrate is a compound used in the treatment of schistosomiasis. It is also used in the textile industry to aid in binding certain dyes to fabrics. It finds application in medicine as an expectorant and a nauseant.
Solubility
Soluble in water. Insoluble in ethanol.
Notes
Incompatible with mineral acids, strong bases, carbonates, lead, silver salts and strong oxidizing agents.
RUO – Research Use Only
General References:
- Vagvala, T. C.; Pandey, S. S.; Ogomi, Y.; Ma, T.; Hayase, S. Investigation of metal xanthates as latent curing catalysts for epoxy resin via formation of in-situ metal sulfides. Inorg. Chim. Acta 2015, 435, 292-298.
- Nguyen, T. H.; Müller, R. H.; Taupitz, M.; Schnorr, J.; Hamm, B.; Wagner, S. Novel oral phosphate binder with nanocrystalline maghemite-phosphate binding capacity and pH effect. Int. J. Pharm. 2015, 482 (1), 21-26.