Search Thermo Fisher Scientific
Thermo Scientific Chemicals
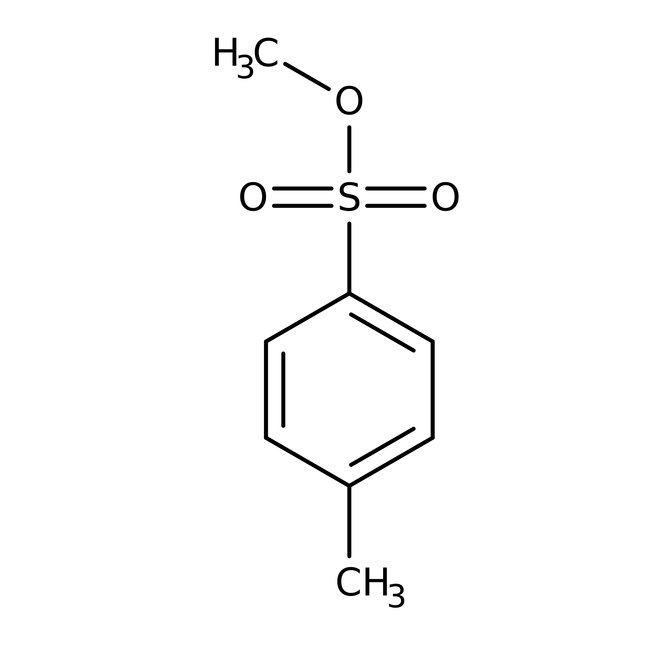
Methyl p-toluenesulfonate, 98%, Thermo Scientific Chemicals
Catalog number ALFA10881.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Chemical Identifiers
CAS609-23-4
IUPAC Name2,4,6-triiodophenol
Molecular FormulaC6H3I3O
InChI KeyVAPDZNUFNKUROY-UHFFFAOYSA-N
SMILESOC1=C(I)C=C(I)C=C1I
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to dark cream to yellow to brown or pale pink
FormPowder
Assay (HPLC)≥97.5%
Identification (FTIR)Conforms
Methyl p-toluenesulfonate is used as a methylating agent in organic synthesis. It acts as a catalyst for alkyd resins. It is also employed in selective 1-substitution reaction of tetrazole. Further, it is used in the preparation of dyes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Methyl p-toluenesulfonate is used as a methylating agent in organic synthesis. It acts as a catalyst for alkyd resins. It is also employed in selective 1-substitution reaction of tetrazole. Further, it is used in the preparation of dyes.
Solubility
Soluble in ether, alcohol, benzene and chloroform. Slightly soluble in petroleum ether. Insoluble in water.
Notes
Store in a cool place. Incompatible with strong oxidizing agents, strong acids and strong bases.
Methyl p-toluenesulfonate is used as a methylating agent in organic synthesis. It acts as a catalyst for alkyd resins. It is also employed in selective 1-substitution reaction of tetrazole. Further, it is used in the preparation of dyes.
Solubility
Soluble in ether, alcohol, benzene and chloroform. Slightly soluble in petroleum ether. Insoluble in water.
Notes
Store in a cool place. Incompatible with strong oxidizing agents, strong acids and strong bases.
RUO – Research Use Only
General References:
- Found to give quantitative alkylation of the lithio-derivatives of enol acetates, whereas alkyl iodides give only modest yields: Synth. Commun., 3, 67 (1973).
- Bouten, P. J.; Lava, K.; van Hest, J.; Hoogenboom, R. Thermal Properties of Methyl Ester-Containing Poly(2-oxazoline)s. Polymers 2015, 7 (10), 1998-2008.
- Zhao, J.; Wang, C.; Zhao, P.; Wen, X.; Lin, C. Bioreducible dextran-polyethylenimine conjugates regulate transgene expression distribution in vivo. J. Mater. Chem. B 2015, 3 (8), 1529-1536.