Search Thermo Fisher Scientific
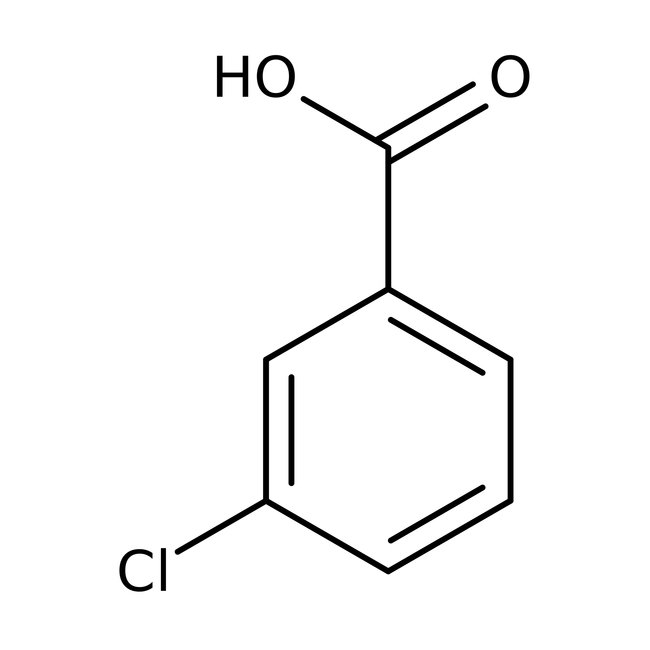
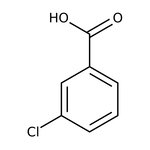
3-Chlorobenzoic acid, 99%, Thermo Scientific Chemicals
| Catalog Number | Quantity |
|---|---|
| ALFA10876.22 | 100 g |
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
A Pseudomonas species which utilized 3-chlorobenzoic acid as a carbon source converted this compound to 3-hydroxybenzoic and 2, 5-dihydroxybenzoic acids. A methanogenic consortium able to use 3-chlorobenzoic acid as its sole energy and carbon source was enriched from anaerobic sewage sludge. A Pseudomonas putida strain 87 capable of assimilating 3-chlorobenzoic acid as a sole source of carbon and energy (3Cba+) was isolated. coupling of 3-chlorobenzoic acid with phenylboronic acid proceeds at room temperature using 0.5 % Pd and at 100°C using 0.1 % Pd to provide the coupled product in 97 % yeild.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
A Pseudomonas species which utilized 3-chlorobenzoic acid as a carbon source converted this compound to 3-hydroxybenzoic and 2, 5-dihydroxybenzoic acids. A methanogenic consortium able to use 3-chlorobenzoic acid as its sole energy and carbon source was enriched from anaerobic sewage sludge. A Pseudomonas putida strain 87 capable of assimilating 3-chlorobenzoic acid as a sole source of carbon and energy (3Cba+) was isolated. coupling of 3-chlorobenzoic acid with phenylboronic acid proceeds at room temperature using 0.5 % Pd and at 100°C using 0.1 % Pd to provide the coupled product in 97 % yeild.
Solubility
Slioghtly soluble in water(0.45 g/L ).
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents.
General References:
- N. Walker, D. Harris. Metabolism of 3-chlorobenzoic acid by Azotobacter species. Soil Biology and Biochemistry. 1970, 2(1), 27-32.
- Pascale Clément,; Dietmar H. Pieper,; Bernardo González. Molecular characterization of a deletion/duplication rearrangement in tfd genes from Ralstonia eutropha JMP134(pJP4) that improves growth on 3-chlorobenzoic acid but abolishes growth on 2,4-dichlorophenoxyacetic acid. Microbiology,. 2001, 147(8), 2141-2148,o.
- The unprotected acid undergoes lithiation at the 2-position with 2.2 moles of s-BuLi in the presence of TMEDA; subsequent reaction with dibromotetrachloroethane provides access to 2-bromo-3-chlorobenzoic acid: Tetrahedron Lett., 36, 881 (1995); Bull. Soc. Chim. Fr., 133, 133 (1996).