Search Thermo Fisher Scientific
Thermo Scientific Chemicals
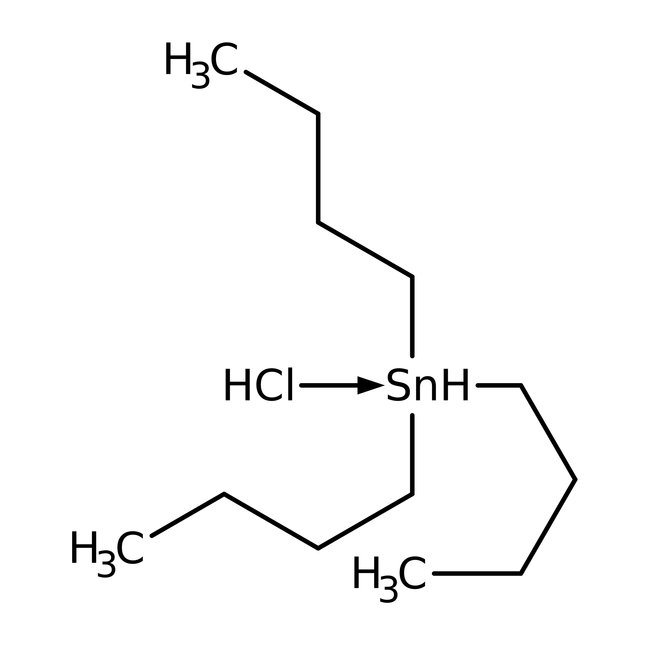
Tri-n-butyltin chloride, 96%, Thermo Scientific Chemicals
CAS: 1461-22-9 | C12H27ClSn | 325.508 g/mol
Catalog number ALFA10746.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Chemical Identifiers
CAS33252-32-3
Specifications Specification Sheet
Specification Sheet
FormCrystals or powder or crystalline powder
Melting Point (clear melt)63.5-69.5?C
Appearance (Color)White to pale cream
Assay (GC)≥96.0%
Tri-n-butyltin chloride is used as an endocrine disruptor as well as an inhibitor for the V-ATPases. It is also used as a rodent-repellent for cable coatings. Further, it is used in hot end glass coating. It also employed as a corrosion remover.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Tri-n-butyltin chloride is used as an endocrine disruptor as well as an inhibitor for the V-ATPases. It is also used as a rodent-repellent for cable coatings. Further, it is used in hot end glass coating. It also employed as a corrosion remover.
Solubility
Miscible with alcohol, heptane, benzene and toluene. Immiscible with water.
Notes
Moisture sensitive. Store in a cool place. Incompatible with strong oxidizing agents.
Tri-n-butyltin chloride is used as an endocrine disruptor as well as an inhibitor for the V-ATPases. It is also used as a rodent-repellent for cable coatings. Further, it is used in hot end glass coating. It also employed as a corrosion remover.
Solubility
Miscible with alcohol, heptane, benzene and toluene. Immiscible with water.
Notes
Moisture sensitive. Store in a cool place. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Reaction with Grignard or lithium reagents gives alkyl or aryl stannanes.
- Organostannanes undergo the Stille coupling reaction, in the presence of Pd catalysts, such as trans-Dichlorobis(triphenyl phosphine) palladium(II) , 10491 , or Tetrakis(triphenyl phosphine) palladium(0) , 10548 , with aryl halides: Angew. Chem. Int. Ed., 25, 508 (1986); J. Org. Chem., 52, 422 (1987), aryl triflates: J. Am. Chem. Soc., 106, 4630 (1984); 108, 3033 (1986); 109, 5478 (1987); Org. Synth. Coll., 9, 553 (1998), or aryl fluorosulfonates: J. Org. Chem., 56, 3493 (1991). LiCl is added in the reaction with triflates to prevent decomposition of the catalyst. These reactions are of wide scope (coupling also occurs with vinyl, allyl or benzyl halides), take place under very mild conditions, and a variety of other functional groups can be tolerated. For recent reviews of the Stille reaction, see: Adv. Met.-Org. Chem., 5, 1 (1996); Org. React., 50, 1 (1997).
- For use in stannylation of allylic chlorides via the ultrasound-promoted Barbier reaction with Mg, see: Chem. Lett., 1857 (1986); Org. Synth. Coll, 9, 707 (1998). For an example of stannylation of indoles as a route to 2-substituted indoles, see 1-Methyl indole, A12605 .
- See also: A. G. Davies, Organotin Chemistry, Wiley-VCH, N. Y. (1997); H. Nozaki in Organometallics in Synthesis, M. Schlosser, Ed., Wiley, N.Y. (1994), p535; (reviews): Tetrahedron, 45, 909 (1989); J. Organomet. Chem., 437, 23 (1992).
- Carrillo, S. G.; Aranda, F. J.; Ortiz, A.; Teruel, J. A. Kinetic characterization of Ca2+-ATPase (SERCA1) inhibition by tri-n-butyltin(IV) chloride. A docking conformation proposal. J. Biomol. Struct. Dyn. 2015, 33 (6), 1211-1224.
- Majetich, G.; Irvin, T. C.; Thompson, S. B. One-pot dehydrations using phenyl isothiocyanate. Tetrahedron Lett. 2015, 56 (23), 3326-3329.