Search Thermo Fisher Scientific
Thermo Scientific Chemicals
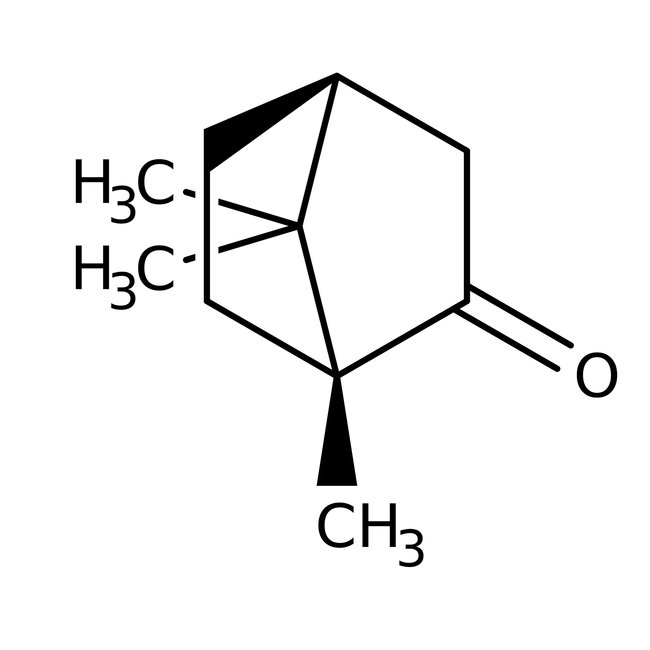
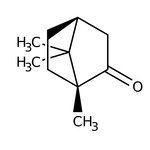
(1R)-(+)-Camphor, 98%, Thermo Scientific Chemicals
CAS: 464-49-3 | C10H16O | 152.24 g/mol
Catalog number ALFA10708.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Chemical Identifiers
CAS1655-07-8
IUPAC Nameethyl 2-oxocyclohexane-1-carboxylate
Molecular FormulaC9H14O3
InChI KeyFGSGHBPKHFDJOP-UHFFFAOYNA-N
SMILESCCOC(=O)C1CCCCC1=O
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless to light yellow
Refractive index1.4775 to 1.4795 (20°C, 589 nm)
Appearance (Form)Liquid
GC>=94 %
Infrared spectrumConforms
(1R)-(+)-Camphor is used as a chiral intermediate and auxiliary. It is also used as a skin antipruritic and as an anti-infective agent.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
(1R)-(+)-Camphor is used as a chiral intermediate and auxiliary. It is also used as a skin antipruritic and as an anti-infective agent.
Solubility
Soluble in water (0.1 g/L at 20°C).
Notes
Keep container tightly sealed. Store in cool, dry conditions in well sealed containers. Incompatible with oxidizing agents.
(1R)-(+)-Camphor is used as a chiral intermediate and auxiliary. It is also used as a skin antipruritic and as an anti-infective agent.
Solubility
Soluble in water (0.1 g/L at 20°C).
Notes
Keep container tightly sealed. Store in cool, dry conditions in well sealed containers. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- G. Grogan.; G. Roberts.; S. Parsons, N. Turner.; S. Flitsch. P450camr, a cytochrome P450 catalysing the stereospecific 6-endo-hydroxylation of (1R)-(+)-camphor. Applied Microbiology and Biotechnology. 2002, 59, (4), 449-454.
- V Santhi.; J.Madhusudana Rao. Asymmetric reduction of prochiral ketones using in situ generated oxazaborolidines derived from amino alcohols of (1R)-camphor as catalysts. Tetrahedron: Asymmetry. 2000, 11 (17), 3553-3560.