Search Thermo Fisher Scientific
Thermo Scientific Chemicals
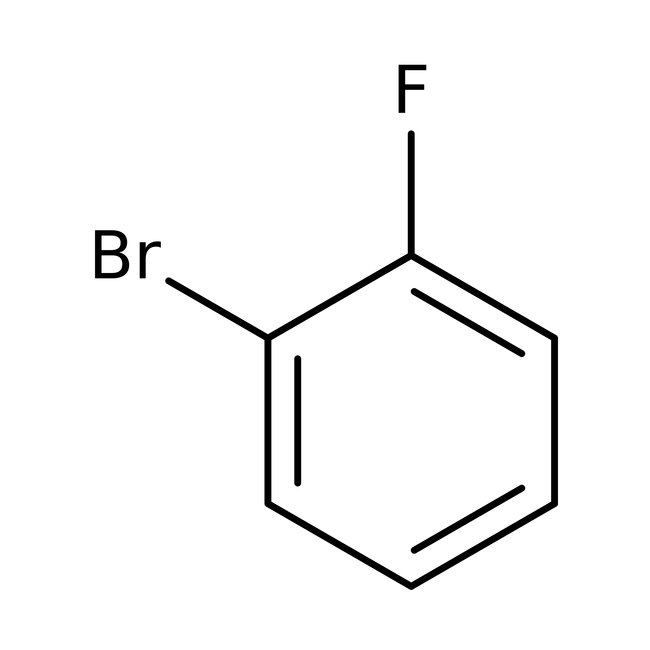
1-Bromo-2-fluorobenzene, 99%, Thermo Scientific Chemicals
CAS: 1072-85-1 | C6H4BrF | 175 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA10635.14 | 25 g |
Catalog number ALFA10635.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or Material1-Bromo-2-fluorobenzene
CAS1072-85-1
Health Hazard 1H226-H315-H319-H335
Health Hazard 2GHS H Statement
H226-H302-H319
Flammable liquid and vapor.
Harmful if swallowed.
Causes serious eye irritation.
H226-H302-H319
Flammable liquid and vapor.
Harmful if swallowed.
Causes serious eye irritation.
Health Hazard 3P210-P233-P235-P240-P241-P242-P243-P261-P264b-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P312-P332+P313-P363-P370+P378q-P501c
View more
1-Bromo-2-fluorobenzene is used as a reactant with cyclopenta-1,3-diene to produce 1,4-dihydro-1,4-methano-naphthalene. This reaction will need reagent Mg, and solvent diethyl ether. The reaction time is 3 hours with the temperature of 20°C,
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-Bromo-2-fluorobenzene is used as a reactant with cyclopenta-1,3-diene to produce 1,4-dihydro-1,4-methano-naphthalene. This reaction will need reagent Mg, and solvent diethyl ether. The reaction time is 3 hours with the temperature of 20°C,
Solubility
Insoluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Stable under recommended storage conditions. Keep away from Strong oxidizing agents .
1-Bromo-2-fluorobenzene is used as a reactant with cyclopenta-1,3-diene to produce 1,4-dihydro-1,4-methano-naphthalene. This reaction will need reagent Mg, and solvent diethyl ether. The reaction time is 3 hours with the temperature of 20°C,
Solubility
Insoluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Stable under recommended storage conditions. Keep away from Strong oxidizing agents .
RUO – Research Use Only
General References:
- KG Rutherford.; W Redmond. 1-Bromo-2-Fluorobenzene. Organic Syntheses. 1963, 100, (1), 54-62.
- M Feuerstein.; H Doucet.; M Santelli. Tetraphosphine/palladium-catalysed Suzuki cross-coupling with sterically hindered aryl bromides and arylboronic acids. Tetrahedron Letters. 2001, 42, (38), 6667-6670.
- On treatment with Mg: J. Org. Chem., 49, 4518 (1984), or n-BuLi: Tetrahedron, 48, 4379 (1992), benzyne is generated, which undergoes [4+2] cycloadditions with furans or pyrroles.
- Whereas n-BuLi effects bromine-metal exchange, lithiation with LDA at -75° results in deprotonation ortho to F; subsequent reaction with electrophiles provides access to mixed dihalo derivatives such as 3-bromo-2-fluorobenzoic acid: Tetrahedron Lett., 36, 881 (1995). Improved yields are obtained by the use of lithium 2,2,6,6-tetramethylpiperidide (LTMP) as base: Tetrahedron Lett., 37, 6551 (1996).