Search Thermo Fisher Scientific
Thermo Scientific Chemicals
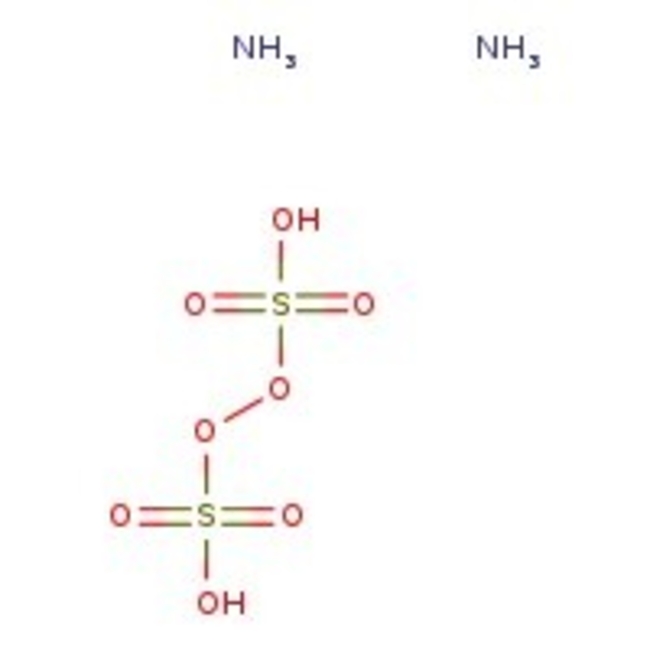
Ammonium peroxydisulfate, 98%, Thermo Scientific Chemicals
CAS: 7727-54-0 | H8N2O8S2 | 228.19 g/mol
Catalog number ALFA10533.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Chemical Identifiers
CAS65719-09-7
IUPAC Namemethyl 2-methylpyridine-3-carboxylate
Molecular FormulaC8H9NO2
InChI KeyKLHWBYHFWALOIJ-UHFFFAOYSA-N
SMILESCOC(=O)C1=C(C)N=CC=C1
View more
Specifications Specification Sheet
Specification Sheet
Refractive Index1.5130-1.5180 @ 20°C
Appearance (Color)Clear colorless to yellow to orange to brown
FormLiquid
Assay (GC)≥96.0%
Ammonium peroxydisulfate is widely utilized as an oxidizing agent and radical initiators in the polymerization of certain alkenes. It is actively involved in in the synthesis of commercially important polymers like styrene-butadiene rubber and polytetrafluoroethylene. It combines with tetramethylethylenediamine to catalyze the polymerization of acrylamide. Due to its oxidizing properties, it can be employed to etch copper on printed circuit boards which is an alternative to ferric chloride solution. Also, it acts as oxidants in organic synthesis.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Ammonium peroxydisulfate is widely utilized as an oxidizing agent and radical initiators in the polymerization of certain alkenes. It is actively involved in in the synthesis of commercially important polymers like styrene-butadiene rubber and polytetrafluoroethylene. It combines with tetramethylethylenediamine to catalyze the polymerization of acrylamide. Due to its oxidizing properties, it can be employed to etch copper on printed circuit boards which is an alternative to ferric chloride solution. Also, it acts as oxidants in organic synthesis.
Solubility
Freely soluble in water.
Notes
Aqueous solution decomposes slowly at room temperature.Moisture sensitive. Incompatible with strong reducing agents, organic materials and powdered metals.
Ammonium peroxydisulfate is widely utilized as an oxidizing agent and radical initiators in the polymerization of certain alkenes. It is actively involved in in the synthesis of commercially important polymers like styrene-butadiene rubber and polytetrafluoroethylene. It combines with tetramethylethylenediamine to catalyze the polymerization of acrylamide. Due to its oxidizing properties, it can be employed to etch copper on printed circuit boards which is an alternative to ferric chloride solution. Also, it acts as oxidants in organic synthesis.
Solubility
Freely soluble in water.
Notes
Aqueous solution decomposes slowly at room temperature.Moisture sensitive. Incompatible with strong reducing agents, organic materials and powdered metals.
RUO – Research Use Only
General References:
- In combination with AgNO3 and Cu(OAc)2, promotes a carbamoyl radical process for the conversion of oxalic acid monoamides to isocyanates: J. Org. Chem., 60, 5430 (1995).
- Hosseini, S. H. Kinetics study of copolymerization of 2-anilinoethanol onto chitosan by ammonium peroxydisulfate as a initiator. Int. J. Phys. Sci. 2015, 10 (4), 142-154.
- Gopalakrishnan, k.; Sultan, S.; Govindaraj, A.; Rao, C. N. R. Supercapacitors based on composites of PANI with nanosheets of nitrogen-doped RGO, BC1.5N, MoS2and WS2. Nano Energy 2015, 12 52-58.

.png-150.jpg)
