Search Thermo Fisher Scientific
Thermo Scientific Chemicals
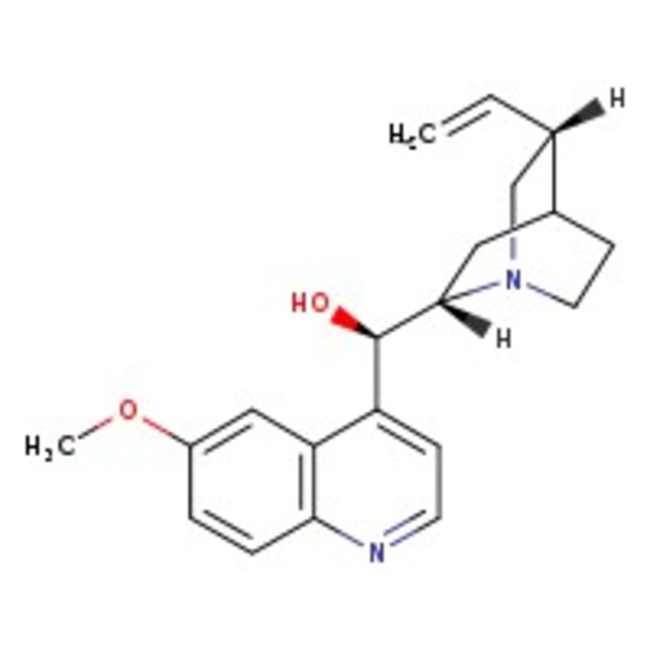
Quinine, anhydrous, 99% (total base), may cont. up to 5% dihydroquinine, Thermo Scientific Chemicals
CAS: 130-95-0 | C20H24N2O2 | 324.42 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA10459.09 | 10 g |
Catalog number ALFA10459.09
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
10 g
Chemical Identifiers
CAS72-17-3
IUPAC Namesodium 2-hydroxypropanoate
Molecular FormulaC3H5NaO3
InChI KeyNGSFWBMYFKHRBD-UHFFFAOYNA-M
SMILES[Na+].CC(O)C([O-])=O
View more
Specifications Specification Sheet
Specification Sheet
Assay (unspecified)58 to 62%
Quinine is used in photochemistry as a common fluorescence standard and as a resolving agent for chiral acids. It is also useful for treating falciparum malaria, lupus, arthritis and vivax malaria. It acts as a flavor component in tonic water and bitter lemon. It is utilized as the chiral moiety for the ligands used in sharpless asymmetric dihydroxylation. Furthermore, it catalyzes the kinetic resolution of furanones.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Quinine is used in photochemistry as a common fluorescence standard and as a resolving agent for chiral acids. It is also useful for treating falciparum malaria, lupus, arthritis and vivax malaria. It acts as a flavor component in tonic water and bitter lemon. It is utilized as the chiral moiety for the ligands used in sharpless asymmetric dihydroxylation. Furthermore, it catalyzes the kinetic resolution of furanones.
Solubility
Soluble in alcohol, chloroform and diethyl ether. Slightly soluble in water and glycerol.
Notes
Incompatible with strong oxidizing agents.
Quinine is used in photochemistry as a common fluorescence standard and as a resolving agent for chiral acids. It is also useful for treating falciparum malaria, lupus, arthritis and vivax malaria. It acts as a flavor component in tonic water and bitter lemon. It is utilized as the chiral moiety for the ligands used in sharpless asymmetric dihydroxylation. Furthermore, it catalyzes the kinetic resolution of furanones.
Solubility
Soluble in alcohol, chloroform and diethyl ether. Slightly soluble in water and glycerol.
Notes
Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Porta, R.; Benaglia, M.; Coccia, F.; Rossi, S.; Puglisi, A. Enantioselective Organocatalysis in Microreactors: Continuous Flow Synthesis of a (S)-Pregabalin Precursor and (S)-Warfarin. Symmetry 2015, 7 (3), 1395-1409.
- Porta, R.; Coccia, F.; Annunziata, R.; Puglisi, A. Comparison of Different Polymer-and Silica-Supported 9-Amino-9-deoxy-epi-quinines as Recyclable Organocatalysts. ChemCatChem 2015, 7 (9), 1490-1499.
- Kessler, S.; González, J.; Vlimant, M.; Glauser, G.; Guerin, P. M. Quinine and artesunate inhibit feeding in the African malaria mosquito Anopheles gambiae: the role of gustatory organs within the mouthparts. Physiol. Entomol. 2014, 39 (2), 172-182.