Search Thermo Fisher Scientific
Thermo Scientific Chemicals
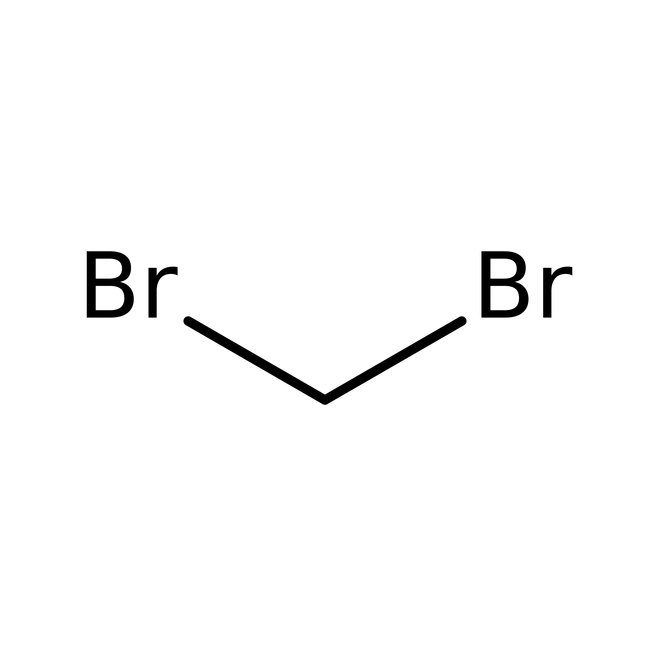
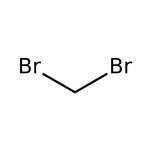
Dibromomethane, 99%, Thermo Scientific Chemicals
CAS: 74-95-3 | CH2Br2 | 173.835 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA10456.36 | 500 g |
Catalog number ALFA10456.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or MaterialDibromomethane
Name NoteStabilized with 50ppm BHT
CAS74-95-3
Health Hazard 1H332
Health Hazard 2GHS H Statement
H332
Harmful if inhaled.
H332
Harmful if inhaled.
View more
Dibromomethane is used as solvent in organic synthesis. It acts as an intermediate in the manufacture of specialty chemicals, agrochemicals and pharmaceuticals. It is useful as extractant and utilized for the determination of 5-nitroimidazoles (5-NDZ) in environmental waters. It is involved in the convertion of catechols to their methylenedioxy derivatives.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Dibromomethane is used as solvent in organic synthesis. It acts as an intermediate in the manufacture of specialty chemicals, agrochemicals and pharmaceuticals. It is useful as extractant and utilized for the determination of 5-nitroimidazoles (5-NDZ) in environmental waters. It is involved in the convertion of catechols to their methylenedioxy derivatives.
Solubility
Miscible with water, chloroform, acetone, ether and alcohol.
Notes
Store in cool place. Incompatible with strong oxidizing agents, aluminum and magnesium.
Dibromomethane is used as solvent in organic synthesis. It acts as an intermediate in the manufacture of specialty chemicals, agrochemicals and pharmaceuticals. It is useful as extractant and utilized for the determination of 5-nitroimidazoles (5-NDZ) in environmental waters. It is involved in the convertion of catechols to their methylenedioxy derivatives.
Solubility
Miscible with water, chloroform, acetone, ether and alcohol.
Notes
Store in cool place. Incompatible with strong oxidizing agents, aluminum and magnesium.
RUO – Research Use Only
General References:
- For in situ formation of dibromomethyllithium by reaction with lithiated 2,2,6,6-Tetramethyl piperidine, A18712, and use in a one-pot ester homologation sequence, safer and more convenient than the classical Arndt-Eistert (diazomethane) method, see: Org. Synth. Coll., 9, 426 (1998):
- Reaction with NaHMDS generates monobromocarbene, which reacts with alkenes to give bromocyclopropanes: Synthesis, 201 (1972). It can also be generated by reaction with diethylzinc in the presence of O2: J. Chem. Soc., Chem. Commun., 364 (1975).
- The Simmons-Smith reaction (see Diiodomethane, A15457) may be extended to the use of dibromomethane in the cyclopropanation of simple electron-deficient alkenes, using a reducing agent prepared from NiBr2, NaI and Zn: Bull. Chem. Soc. Jpn., 56, 1025 (1983); or with CuCl/Zn: J. Org. Chem., 55, 2491 (1990).
- With TiCl4/Zn, methylenation of ketones occurs, as a useful mild alternative to the Wittig reaction: Tetrahedron Lett., 23, 4293 (1982); Org. Synth. Coll., 8, 386 (1993). For a variant using Zn, CuCl and catalytic TiCl4, see: J. Org. Chem., 54, 2388 (1989).
- With alcohols, formaldehyde acetals can be formed under phase-transfer conditions: Bull. Chem. Soc. Jpn., 66, 2149 (1993). Diols give methylenedioxy derivatives, which are components of many biologically-important molecules. For use of KF in DMF as base in the methylenation of catechols, see: Tetrahedron Lett., 3361 (1976). KOH in DMSO has been recommended for the methylenation of base-stable carbohydrate diols: Synthesis, 421 (1982). For the methylenation of ribonucleosides under phase-transfer conditions, see: Synthesis, 715 (1981).