Search Thermo Fisher Scientific
Thermo Scientific Chemicals
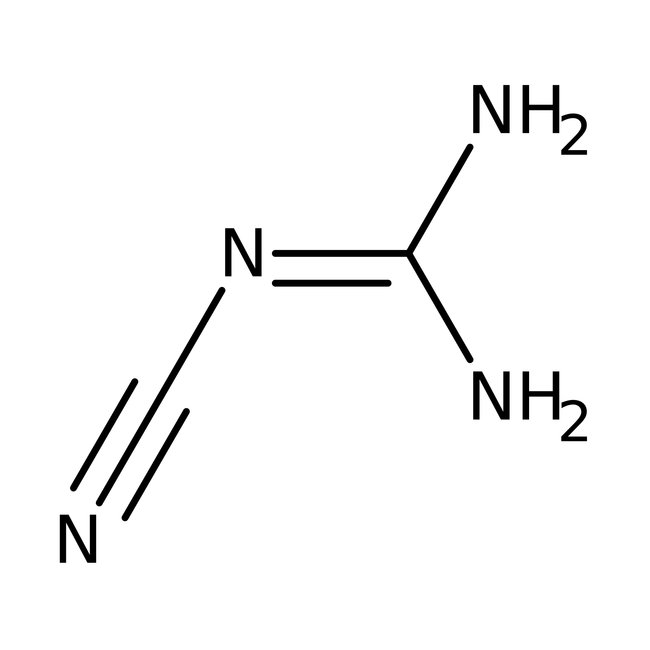
Dicyandiamide, 99%, Thermo Scientific Chemicals
CAS: 461-58-5 | C2H4N4 | 84.08 g/mol
Catalog number ALFA10451.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Chemical Identifiers
CAS107021-38-5
IUPAC Name2-[(5-bromo-4-chloro-1H-indol-3-yl)oxy]-6-(hydroxymethyl)oxane-3,4,5-triol
Molecular FormulaC14H15BrClNO6
InChI KeyOPIFSICVWOWJMJ-UHFFFAOYNA-N
SMILESOCC1OC(OC2=CNC3=CC=C(Br)C(Cl)=C23)C(O)C(O)C1O
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White
Water Content (Karl Fischer Titration)≤1.0%
Optical Rotation+147° ±2° (c=1, 1:1 DMF:water)
FormCrystals or powder or crystalline powder
Proton NMRConforms to structure
View more
Dicyandiamide is used in the synthesis of barbiturates. It is used as a stabilizer of ammonium dinitramide melt. It is used as hardener.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Dicyandiamide is used in the synthesis of barbiturates. It is used as a stabilizer of ammonium dinitramide melt. It is used as hardener.
Solubility
Soluble in Water (32g/L ) at 20°C
Notes
Store at 2-8°C. Incompatible with strong acids, strong oxidizing agents, strong acids, strong bases, ammonium nitrate, potassium chlorates.
Dicyandiamide is used in the synthesis of barbiturates. It is used as a stabilizer of ammonium dinitramide melt. It is used as hardener.
Solubility
Soluble in Water (32g/L ) at 20°C
Notes
Store at 2-8°C. Incompatible with strong acids, strong oxidizing agents, strong acids, strong bases, ammonium nitrate, potassium chlorates.
RUO – Research Use Only
General References:
- A Amberger. Research on dicyandiamide as a nitrification inhibitor and future outlook. Communications in Soil Science & Plant Analysis.1989, 20, 1933-1955.
- Reacts with aromatic nitriles in the presence of base to give 6-aryl-1,3,5-triazines (benzoguanamines); see, e.g.: Org. Synth. Coll., 4, 78 (1963).