Search Thermo Fisher Scientific
Thermo Scientific Chemicals
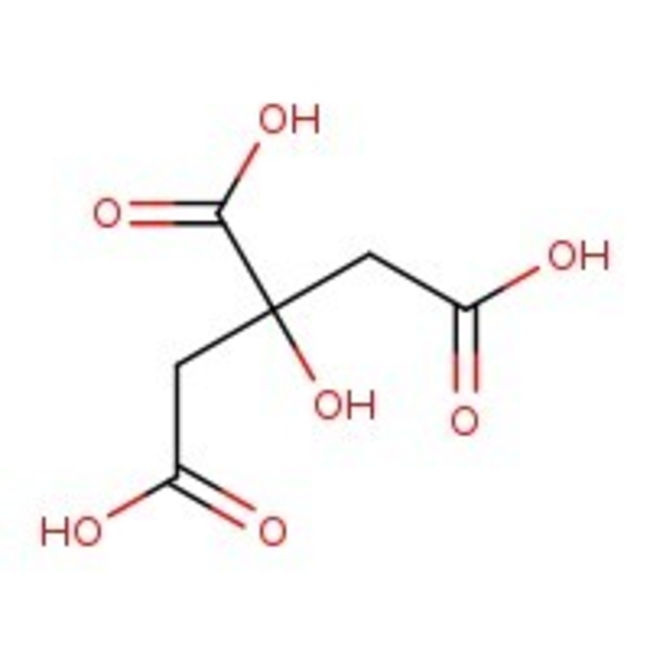
Citric acid, 99+%, Thermo Scientific Chemicals
CAS: 77-92-9 | C6H8O7 | 192.12 g/mol
Catalog number ALFA10395.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Chemical Identifiers
CAS7790-69-4
IUPAC Namelithium(1+) nitrate
Molecular FormulaLiNO3
InChI KeyIIPYXGDZVMZOAP-UHFFFAOYSA-N
SMILES[Li+].[O-][N+]([O-])=O
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Form)Adhering crystalline powder
Titration after Ion exchange>=99.0 %
Appearance (Color)White
Loss on drying=<1 % (200°C)
Citric acid is the most widely used organic acid and pH-control agent in foods, beverages, pharmaceuticals and technical applications. Used in pharmaceutical preparations, especially in effervescent tablets. It is used as a natural preservative.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Citric acid is the most widely used organic acid and pH-control agent in foods, beverages, pharmaceuticals and technical applications. Used in pharmaceutical preparations, especially in effervescent tablets. It is used as a natural preservative.
Solubility
Soluble in water, alcohol, ether, dimethylsufoxide and ethylacetate.
Notes
Hygroscopic. Incompatible with alkalies, bases, metal nitrates and oxidizing agents.
Citric acid is the most widely used organic acid and pH-control agent in foods, beverages, pharmaceuticals and technical applications. Used in pharmaceutical preparations, especially in effervescent tablets. It is used as a natural preservative.
Solubility
Soluble in water, alcohol, ether, dimethylsufoxide and ethylacetate.
Notes
Hygroscopic. Incompatible with alkalies, bases, metal nitrates and oxidizing agents.
RUO – Research Use Only
General References:
- Pan, K.; Zhong, Q. Improving Clarity and Stability of Skim Milk Powder Dispersions by Dissociation of Casein Micelles at pH 11.0 and Acidification with Citric Acid. Agric. Food Chem. 2013, 61 (38), 9260-9268.
- Kolah, A. K.; Asthana, N. S.; Vu, D. T.; Carl T. Lira.; Miller, D. J. Reaction Kinetics of the Catalytic Esterification of Citric Acid with Ethanol. Ind. Eng. Chem. Res. 2007, 46 (10), 3180-3187.
- Gogoi, P.; Boruah, M.; Sharma, S.; Dolui, S. K. Blends of Epoxidized Alkyd Resins Based on Jatropha Oil and the Epoxidized Oil Cured with Aqueous Citic Acid Solution: A Green Technology Approach. ACS Sustainable Chem. Eng. 2015, 3 (2), 261-268.