Search Thermo Fisher Scientific
Thermo Scientific Chemicals
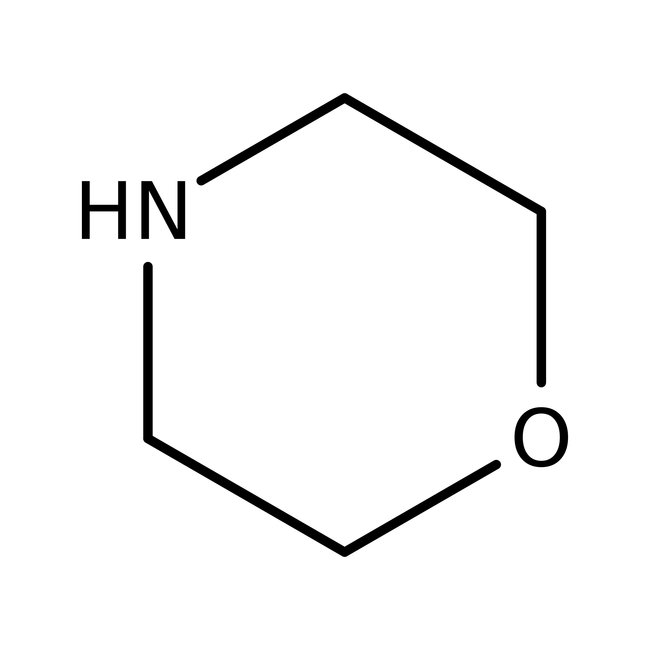
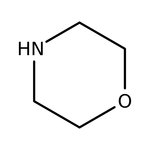
Morpholine, 99%, Thermo Scientific Chemicals
CAS: 110-91-8 | C4H9NO | 87.12 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA10355.36 | 500 g |
Catalog number ALFA10355.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or MaterialMorpholine
CAS110-91-8
Health Hazard 1H226-H302-H311+H331-H314-H335-H373
Health Hazard 2GHS H Statement
H314-H226-H302-H312-H332
H314-H226-H302-H312-H332
Health Hazard 3P210-P233-P235-P240-P241-P242-P243-P260-P264b-P270-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P370+P378q-P501c
View more
Morpholine is a common additive for pH adjustment in both fossil fuel and nuclear power plant steam systems. It is widely used in organic synthesis as a building block in the preparation of the antibiotic linezolid, the anticancer agent gefitinib and the analgesic dextromoramide. It is also used as a solvent in chemical reactions and in the preparation of enamines. Further, it serves as a chemical emulsifier in the process of waxing fruit.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Morpholine is a common additive for pH adjustment in both fossil fuel and nuclear power plant steam systems. It is widely used in organic synthesis as a building block in the preparation of the antibiotic linezolid, the anticancer agent gefitinib and the analgesic dextromoramide. It is also used as a solvent in chemical reactions and in the preparation of enamines. Further, it serves as a chemical emulsifier in the process of waxing fruit.
Solubility
Miscible with water.
Notes
Hygroscopic. Incompatible with strong oxidizing agents.
Morpholine is a common additive for pH adjustment in both fossil fuel and nuclear power plant steam systems. It is widely used in organic synthesis as a building block in the preparation of the antibiotic linezolid, the anticancer agent gefitinib and the analgesic dextromoramide. It is also used as a solvent in chemical reactions and in the preparation of enamines. Further, it serves as a chemical emulsifier in the process of waxing fruit.
Solubility
Miscible with water.
Notes
Hygroscopic. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Reagent for the cleavage of Fmoc protecting groups in peptide synthesis (see 9-Fluorenyl methyl chloroformate, A11683): J. Am. Chem. Soc., 92, 5748 (1970); J. Org. Chem., 37, 3404 (1972). See Appendix 6 for peptide reagents.
- In the Kindler modification of the Willgerodt reaction, aryl ketones are converted to ω-arylalkanoic acids by reaction with sulfur and an amine, usually morpholine: Org. Synth. Coll., 9, 99 (1998); reviews: Org. React., 3, 83 (1946); Synthesis, 358 (1975).
- Lithium morpholide adds to aromatic aldehydes at low temperature, a useful means for the protection of the aldehyde group in lithiations: Tetrahedron Lett., 25, 4213 (1981); J. Org. Chem., 48, 2356 (1983).
- Chlorination with hypochlorite gives N-chloromorpholine, a useful reagent in strongly acid media for the selective chlorination of activated aromatics: Org. Synth. Coll., 8, 167 (1993), and references therein.
- Chen, D.; Miao, H.; Zou, J.; Cao, P.; Ma, N.; Zhao, Y.; Wu, Y. Novel Dispersive Micro-Solid-Phase Extraction Combined with Ultrahigh-Performance Liquid Chromatography-High-Resolution Mass Spectrometry To Determine Morpholine Residues in Citrus and Apples. J. Agric. Food. Chem. 2015, 63 (2), 485-492.
- Ye, L.; Zhai, L.; Fang, J.; Liu, J.; Li, C.; Guan, R. Synthesis and characterization of novel cross-linked quaternized poly (vinyl alcohol) membranes based on morpholine for anion exchange membranes. Solid State Ionics 2013, 240, 1-9.