Search Thermo Fisher Scientific
Thermo Scientific Chemicals
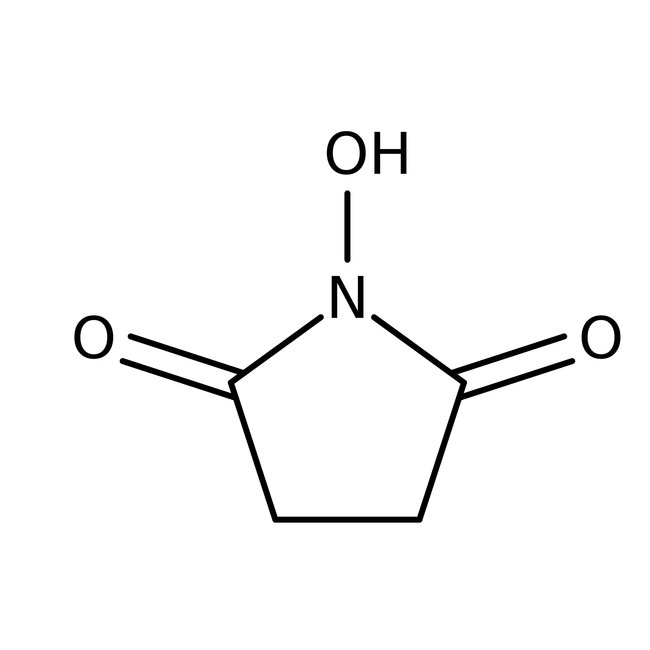
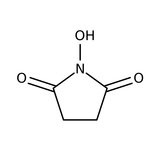
N-Hydroxysuccinimide, 98+%, Thermo Scientific Chemicals
CAS: 6066-82-6 | C4H5NO3 | 115.088 g/mol
Catalog number ALFA10312.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS109-65-9
IUPAC Name1-bromobutane
Molecular FormulaC4H9Br
InChI KeyMPPPKRYCTPRNTB-UHFFFAOYSA-N
SMILESCCCCBr
View more
Specifications Specification Sheet
Specification Sheet
Assay (GC)≥98.0%
Identification (FTIR)Conforms
Appearance (Color)Clear colorless to pale yellow
Refractive Index1.4380-1.4420 @ 20?C
FormLiquid
N-Hydroxysuccinimide is used for improved amidations in the carbodiimide method. It is also used to activate a carboxyl group and reacts with amine to form amide. It is involved in the preparation of N-hydroxymaleimide-styrene copolymer. Further, it finds use in analytical chemistry. As an additive, it is used in the carbodiimide method for improved amidations and peptide couplings.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
N-Hydroxysuccinimide is used for improved amidations in the carbodiimide method. It is also used to activate a carboxyl group and reacts with amine to form amide. It is involved in the preparation of N-hydroxymaleimide-styrene copolymer. Further, it finds use in analytical chemistry. As an additive, it is used in the carbodiimide method for improved amidations and peptide couplings.
Solubility
Soluble in water, dimethyl formamide, alcohols and ethyl acetate. Insoluble in cold ether.
Notes
Hygroscopic. Moisture sensitive. Incompatible with strong oxidizing agents, acid chlorides, acid anhydrides and strong bases. Store in a cool place.
N-Hydroxysuccinimide is used for improved amidations in the carbodiimide method. It is also used to activate a carboxyl group and reacts with amine to form amide. It is involved in the preparation of N-hydroxymaleimide-styrene copolymer. Further, it finds use in analytical chemistry. As an additive, it is used in the carbodiimide method for improved amidations and peptide couplings.
Solubility
Soluble in water, dimethyl formamide, alcohols and ethyl acetate. Insoluble in cold ether.
Notes
Hygroscopic. Moisture sensitive. Incompatible with strong oxidizing agents, acid chlorides, acid anhydrides and strong bases. Store in a cool place.
RUO – Research Use Only
General References:
- Reagent for the preparation of active N-hydroxysuccinimide esters for peptide coupling with minimum racemization: J. Am. Chem. Soc., 86, 1839 (1964), or as an additive in the DCC method (see N,N'-Dicyclohexyl carbodiimide, A10973 ) to suppress racemization: J. Am. Chem. Soc., 89, 7151 (1967). For peptide reagents, see Appendix 6.
- For a study of the aminolysis of N-hydroxysuccinimide esters, see: J. Org. Chem., 53, 3583 (1988).
- Park, C.; Vo, C. L. N.; Kang, T.; Oh, E.; Lee, B. J. New method and characterization of self-assembled gelatin-oleic nanoparticles using a desolvation method via carbodiimide-hydroxysuccinimide (EDCHS) reaction. Eur. J. Pharm. Biopharm. 2015, 89, 365-373.
- Peng, Z.; McGee, W. M.; Bu, J.; Barefoot, N. Z.; McLuckey, S. A. Gas phase reactivity of carboxylates with N-hydroxysuccinimide esters. J. Am. Soc. Mass. Spectrom. 2015, 26 (1), 174-180.