Search Thermo Fisher Scientific
Thermo Scientific Chemicals
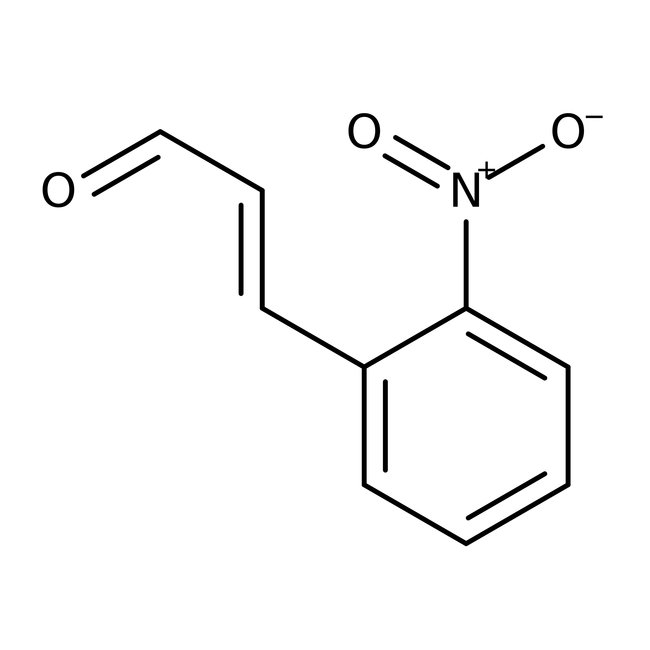
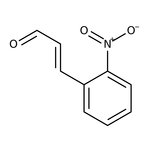
2-Nitrocinnamaldehyde, predominantly trans, 98%, Thermo Scientific Chemicals
CAS: 1466-88-2 | C9H7NO3 | 177.159 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA10308.09 | 10 g |
Catalog number ALFA10308.09
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
10 g
Specifications
Chemical Name or Material2-Nitrocinnamaldehyde
Name Notepredominantly trans
CAS1466-88-2
Health Hazard 1H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
View more
It finds its uses as a actinometer for the UV-A range of photostability testing of pharmaceuticals. 2-Nitrocinnamaldehyde can be oxidized to 2-nitrocinnamic acid which can be used in the Baeyer-Emmerling indole synthesis to produce indole and substituted indoles.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It finds its uses as a actinometer for the UV-A range of photostability testing of pharmaceuticals. 2-Nitrocinnamaldehyde can be oxidized to 2-nitrocinnamic acid which can be used in the Baeyer-Emmerling indole synthesis to produce indole and substituted indoles.
Solubility
Soluble in methanol. Insoluble in water.
Notes
Air sensitive. Store away from oxidizing agents and air. Keep the container tightly closed and place it in a cool, dry and well ventilated condition. Store under inert gas.
It finds its uses as a actinometer for the UV-A range of photostability testing of pharmaceuticals. 2-Nitrocinnamaldehyde can be oxidized to 2-nitrocinnamic acid which can be used in the Baeyer-Emmerling indole synthesis to produce indole and substituted indoles.
Solubility
Soluble in methanol. Insoluble in water.
Notes
Air sensitive. Store away from oxidizing agents and air. Keep the container tightly closed and place it in a cool, dry and well ventilated condition. Store under inert gas.
RUO – Research Use Only
General References:
- Yoshiyasu Kitahara, et al. Synthesis of meridine, cystodamine, and related compounds including iminoquinolinequinone structure.Tetrahedron.,1998,54(29), 8421-8432.
- Kengo Akagawa, et al. Friedel-Crafts-type alkylation in aqueous media using resin-supported peptide catalyst having polyleucine.Tetrahedron Letters.,2009,50(40), 5602-5604.