Search Thermo Fisher Scientific
Thermo Scientific Chemicals
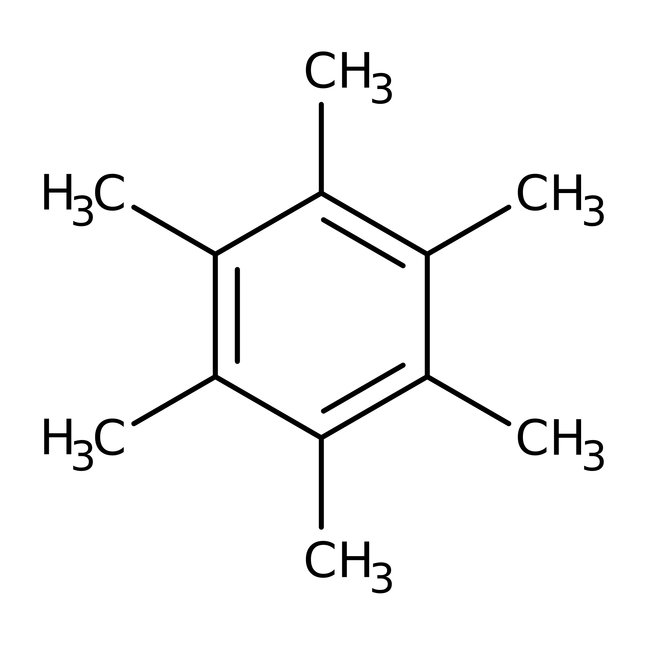
Hexamethylbenzene, 99+%, Thermo Scientific Chemicals
CAS: 87-85-4 | C12H18 | 162.276 g/mol
Catalog number ALFA10294.06
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
5 g
Specifications
Chemical Name or MaterialHexamethylbenzene
CAS87-85-4
Melting Point163°C to 167°C
Recommended StorageAmbient temperatures
Density1.063
View more
Hexamethylbenzene is used as pharmaceutical intermediates, in organic synthesis of compounds, as a solvent for He-NMR spectroscopy. Used as a ligand in organometallic chemistry. Reaction with dimethyldioxirane, gives the major product, an unusual oxepane triepoxide.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Hexamethylbenzene is used as pharmaceutical intermediates, in organic synthesis of compounds, as a solvent for He-NMR spectroscopy. Used as a ligand in organometallic chemistry. Reaction with dimethyldioxirane, gives the major product, an unusual oxepane triepoxide.
Solubility
Insoluble in water
Notes
Incompatible with nitromethane, strong oxidizing agents.
Hexamethylbenzene is used as pharmaceutical intermediates, in organic synthesis of compounds, as a solvent for He-NMR spectroscopy. Used as a ligand in organometallic chemistry. Reaction with dimethyldioxirane, gives the major product, an unusual oxepane triepoxide.
Solubility
Insoluble in water
Notes
Incompatible with nitromethane, strong oxidizing agents.
RUO – Research Use Only
General References:
- Helena L. Chum; V. R. Koch; L. L. Miller; R. A. Osteryoung. Electrochemical scrutiny of organometallic iron complexes and hexamethylbenzene in a room temperature molten salt. J. Am. Chem. Soc.1975, 97 (11), 3264-3265.
- Oxidative rearrangement occurs on treatment with peroxytrifluoroacetic acid (caution! 90% H2O2) and BF3 etherate to give hexamethyl-2,4-cyclohexadienone: J. Am. Chem. Soc., 88, 1005 (1966); Org. Synth. Coll., 5, 598 (1973). Reaction with dimethyldioxirane, on the other hand, gives, as the major product, an unusual oxepane triepoxide: J. Org. Chem., 61, 7660 (1996):