Search Thermo Fisher Scientific
Thermo Scientific Chemicals
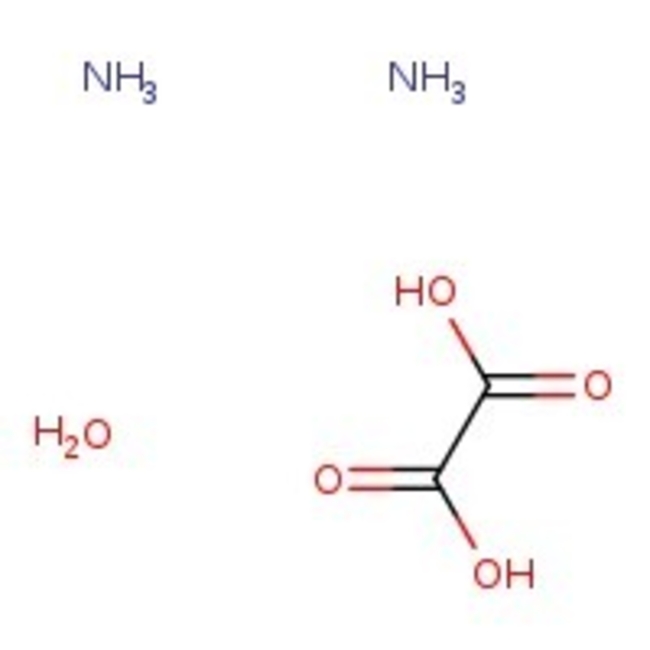
Ammonium oxalate monohydrate, 98%, Thermo Scientific Chemicals
CAS: 6009-70-7 | C2H10N2O5 | 142.111 g/mol
Catalog number ALFA10263.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Chemical Identifiers
CAS1122-41-4
IUPAC Name(2,4-dichlorophenyl)sulfanide
Molecular FormulaC6H3Cl2S
InChI KeyFGBVJFREPSJSNG-UHFFFAOYSA-M
SMILES[S-]C1=CC=C(Cl)C=C1Cl
View more
Specifications Specification Sheet
Specification Sheet
Assay (GC)≥96.0%
FormLiquid
Refractive Index1.6150-1.6200 @ 20?C
Appearance (Color)Clear colorless
Ammonium oxalate monohydrate is widely utilized as a buffering reagent and a dispersant to determine the surface interaction of platelets. It finds an application to study its acute poisoning effect on sheep and to investigate the formation of oxalate monoalkylamide in the human lens. It is also used in the detection and determination of calcium, lead, fluoride and rare earth metals. It is employed as chelators and forms complexes with metals. It acts as reducing agent in gold extraction and is an active ingredient of ferrous metal surface polishing fluid. It is a promoting agent in production of cobalt oxide and nickel oxide.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Ammonium oxalate monohydrate is widely utilized as a buffering reagent and a dispersant to determine the surface interaction of platelets. It finds an application to study its acute poisoning effect on sheep and to investigate the formation of oxalate monoalkylamide in the human lens. It is also used in the detection and determination of calcium, lead, fluoride and rare earth metals. It is employed as chelators and forms complexes with metals. It acts as reducing agent in gold extraction and is an active ingredient of ferrous metal surface polishing fluid. It is a promoting agent in production of cobalt oxide and nickel oxide.
Solubility
Soluble in water. Slightly soluble in alcohol.
Notes
Incompatible with strong oxidizing agents and strong acids.
Ammonium oxalate monohydrate is widely utilized as a buffering reagent and a dispersant to determine the surface interaction of platelets. It finds an application to study its acute poisoning effect on sheep and to investigate the formation of oxalate monoalkylamide in the human lens. It is also used in the detection and determination of calcium, lead, fluoride and rare earth metals. It is employed as chelators and forms complexes with metals. It acts as reducing agent in gold extraction and is an active ingredient of ferrous metal surface polishing fluid. It is a promoting agent in production of cobalt oxide and nickel oxide.
Solubility
Soluble in water. Slightly soluble in alcohol.
Notes
Incompatible with strong oxidizing agents and strong acids.
RUO – Research Use Only
General References:
- Kripal, R.; Yadav, A. K. Theoretical calculation of zero field splitting parameters of Cr3+doped ammonium oxalate monohydrate. Physica B. 2015, 466, 16-18.
- Qiao, Y.; Wang, K.; Yuan, H.; Yang, K.; Zou, B. Negative Linear Compressibility in Organic Mineral Ammonium Oxalate Monohydrate with Hydrogen Bonding Wine-Rack Motifs. J. Phys. Chem. Lett. 2015, 6 (14), 2755-2760.