Search Thermo Fisher Scientific
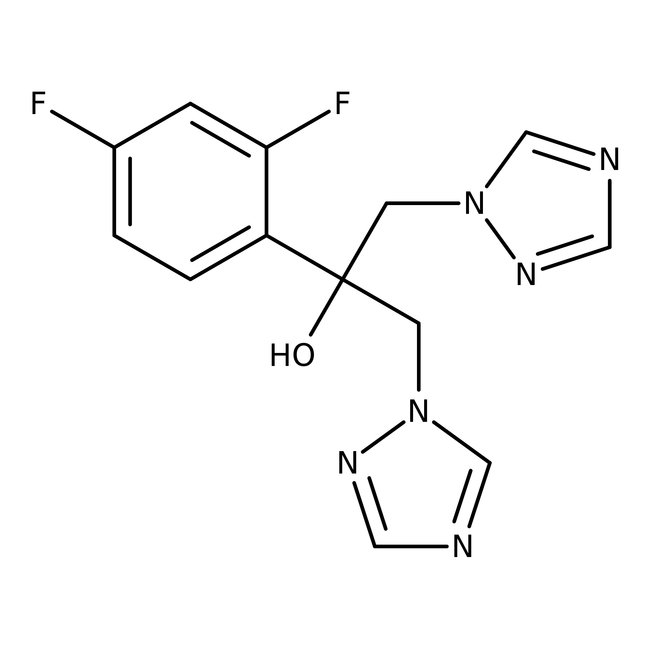
Fluconazole, 98%
General Description
• Fluconazole, 98% is a triazole compound showing antifungal activity mainly against Candida, Coccidioides, and Cryptococcus species
• It has been shown to inhibit fungal cytochrome P-450 sterol C-14 α-demethylation, resulting in the accumulation of fungal 14 α-methyl sterols, the loss of normal fungal sterols, and associated fungistatic activity
• It also inhibits cytochrome P450 family 2 subfamily c member 9 (CYP2C9)
• Mammalian cell demethylation is much less susceptible to fluconazole inhibition
Applications
• Fluconazole has fungistatic activity against many fungal strains including Candida albicans, Candida glabrata, Candida parapsilosis, Candida tropicalis, and Cryptococcus neoformans.
• Fluconazole interferes with fungal ergosterol synthesis and downregulates the metallothionein gene
• In biological experiments, this compound has been used to assess minimum inhibitory concentration in (MIC)-fungal assays
• It has also been used as a positive control in antifungal assay of plants
General References:
- Clancy, C.J.; Yu, V.L.; Morris, A.J.; Snydman, D.R.; Nguyen, M.H. Fluconazole MIC and the fluconazole dose/MIC ratio correlate with therapeutic response among patients with candidemia. Antimicrob Agents Chemother. 2005, 49 (8), 3171-3177.
- Matsui, K.; Tsume, Y.; Amidon, G. E.; Amidon, G. L. In Vitro Dissolution of Fluconazole and Dipyridamole in Gastrointestinal Simulator (GIS), Predicting in Vivo Dissolution and Drug-Drug Interaction Caused by Acid-Reducing Agents. Mol. Pharmaceutics. 2015, 12 (7), 2418-2428.
- Bhardwaj, T.; Bhardwaj, V.; Sharma, K.; Gupta, A.; Cameotra, S. S.; Sharma, P. Thermo-acoustical analysis of sodium dodecyl sulfate: Fluconazole (antifungal drug) based micellar system in hydro-ethanol solutions for potential drug topical application. J. Chem. Thermodyn. 2014, 78, 1-6.